Effects of Acid Rain on Materials
Not all acidic deposition is wet. Sometimes dust particles can become acidic as well, and this is called dry deposition. When acid rain and dry acidic particles fall to earth, the nitric and sulfuric acid that make the particles acidic can land on statues, buildings, and other manmade structures, and damage their surfaces. The acidic particles corrode metal and cause paint and stone to deteriorate more quickly. They also dirty the surfaces of buildings and other structures such as monuments.
The consequences of this damage can be costly:
- damaged materials that need to be repaired or replaced,
- increased maintenance costs, and
- loss of detail on stone and metal statues, monuments and tombstones.
Other Effects of SO2 and NOX
Visibility
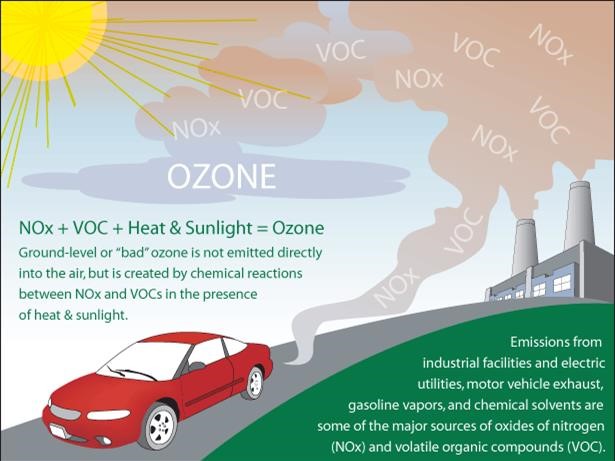
In the atmosphere, SO2 and NOX gases can be transformed into sulfate and nitrate particles, while some NOX can also react with other pollutants to form ozone. These particles and ozone make the air hazy and difficult to see through. This affects our enjoyment of national parks that we visit for the scenic view such as Shenandoah and the Great Smoky Mountains.
Walking in acid rain, or even swimming in a lake affected by acid rain, is no more dangerous to humans than walking in normal rain or swimming in non-acidic lakes. However, when the pollutants that cause acid rain —SO2 and NOX, as well as sulfate and nitrate particles— are in the air, they can be harmful to humans.
SO2 and NOX react in the atmosphere to form fine sulfate and nitrate particles that people can inhale into their lungs. Many scientific studies have shown a relationship between these particles and effects on heart function, such as heart attacks resulting in death for people with increased heart disease risk, and effects on lung function, such as breathing difficulties for people with asthma.
Learn more about:
- Sulfur Dioxide
- Nitrogen Oxides
- Particulate Matter (PM)
- Asthma
In addition, NOX emissions also contribute to ground level ozone, which is also harmful to human health.