Air Pollution Particulate Matter
1. What is Particulate Matter (PM)?
- 1.1 Why does particle size matter
- 1.2 How are particles formed?
- 1.3 Which materials are the main components of particulate matter?
Particulate matter is the sum of all solid and liquid particles suspended in air many of which are hazardous. This complex mixture includes both organic and inorganic particles, such as dust, pollen, soot, smoke, and liquid droplets. These particles vary greatly in size, composition, and origin.
Particles in air are either:
- directly emitted, for instance when fuel is burnt and when dust is carried by wind, or
- indirectly formed, when gaseous pollutants previously emitted to air turn into particulate matter.
1.1 Why does particle size matter
The aerodynamic properties of particles determine how they are transported in air and how they can be removed from it. These properties also govern how far they get into the air passages of therespiratory system. Additionally, they provide information on the chemical composition and the sources of particles.
Particles have irregular shapes and their aerodynamic behaviour is expressed in terms of the diameter of an idealised sphere. The sampling and description of particles is based on thisaerodynamic diameter, which is usually simply referred to as ‘particle size’. Particles having the same aerodynamic diameter may have different dimensions and shapes. Some airborne particles are over 10,000 times bigger than others in terms of aerodynamic diameter.
Based on size, particulate matter is often divided into two main groups:
- The coarse fraction contains the larger particles with a size ranging from 2.5 to 10 µm (PM10 - PM2.5).
- The fine fraction contains the smaller ones with a size up to 2.5 µm (PM2.5). The particles in the fine fraction which are smaller than 0.1 µm are called ultrafine particles.
Most of the total mass of airborne particulate matter is usually made up of fine particles ranging from 0.1 to 2.5 µm. Ultrafine particles often contribute only a few percent to the total mass, though they are the most numerous, representing over 90% of the number of particles.
1.2 How are particles formed?
Coarse particles are produced by the mechanical break-up of larger solid particles. The coarse fraction can include dust from roads, agricultural processes, uncovered soil or mining operations, as well as non-combustible materials released when burning fossil fuels. Pollen grains, mould spores, and plant and insect parts can also contribute to the coarse fraction. Finally, evaporation of sea spray can produce large particles near coasts.
Fine particles are largely formed from gases. Ultrafine particles (up to 0.1 µm) are formed by nucleation, which is the initial stage in which gas becomes a particle. These particles can grow up to a size of 1 µm either through condensation, when additional gas condensates on the particles, or through coagulation, when two or more particles combine to form a larger particle. Particles produced by the intermediate reactions of gases in the atmosphere are called secondary particles.
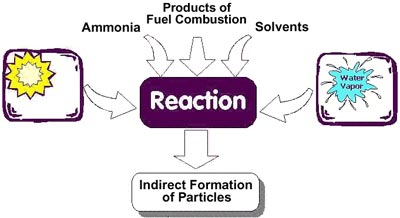
Source: US EPA www.epa.gov/urbanair/pm/
Combustion of fossil fuels such as coal, oil, and petrol can produce
- coarse particles from the release of non-combustible materials such as fly ash,
- fine particles from the condensation of materials vaporized during combustion, and
- secondary particles through the atmospheric reactions of sulphur oxides and nitrogen oxides initially released as gases.
1.3 Which materials are the main components of particulate matter?
On average, the two main components of particulate matter in Europe are sulphate and organicmatter. This is true both for fine particles (PM2.5) and for coarse and fine particles combined (PM10).
However, near roads mineral dust is also a main component of PM10.
On days when the levels of particulate matter in the air are high (PM10 exceeds 50 µg/m3), nitrate is also a major component of both PM10 and PM2.5.
Soot, also referred to as black carbon, makes up 5 to10% of fine particles and somewhat less of coarse particles; near certain roads the proportion of soot can reach 15 to 20%
1.3 Which materials are the main components of particulate matter?
The source document for this Digest states:
Recently a comprehensive report on PM phenomology in Europe was compiled (7). Sulfate and organic matter are the two main contributors to the annual average PM10 andPM2.5 mass concentrations, except at kerbside sites where mineral dust (including trace elements) is also a main contributor to PM10. On days when PM10 > 50 µg/m3, nitrate becomes also a main contributors to PM10 and PM2.5. Black carbon contributes 5–10% toPM2.5 and somewhat less to PM10 at all sites, including the natural background sites. Its contribution increases to 15–20% at some of the kerbside sites. Because of its complexity and the importance of particle size in determining exposure and human dose, numerous terms are used to describe particulate matter. Some are derived from and defined by sampling and/or analytic methods, e.g. “suspended particulate matter”, “total suspended particulates”, “black smoke”. Others refer more to the site of deposition in the respiratory tract, e.g. “inhalable particles”, which pass into the upper airways (nose and mouth), and “thoracic particles”, which deposit within the lower respiratory tract, and “respirable particles”, which penetrate to the gas-exchange region of the lungs. Other terms, such as “PM10”, have both physiological and sampling connotations.


No comments:
Post a Comment