What is the pH of lubricating oil?
Additive depletion, contamination and oxidation are common pathways of lubricant degradation. The acid number (AN) test is one of the methods available in the oil analysis field used to estimate the amount of additive depletion, acidic contamination and oxidation. AN does not directly measure the rate of oxidation, it merely measures the by-product of oxidation. It is also beneficial to trend AN to determine the rate of depletion of certain additives. The purpose of this article is to attempt to answer the following questions:
What are the main objectives of measuring AN?
What nomenclature is being used in industry? (strong acid number (SAN), total acid number (TAN), etc.)?
What standardized methods are currently used in the industry?
What modified tests exist and why?
What are the advantages and disadvantages of each test (reproducibility, repeatability, etc.)?
What are the dos and don'ts of comparing results?
How is AN trended and what are the common trends?
Once these questions are answered, a better understanding of how to use AN results will be achieved.
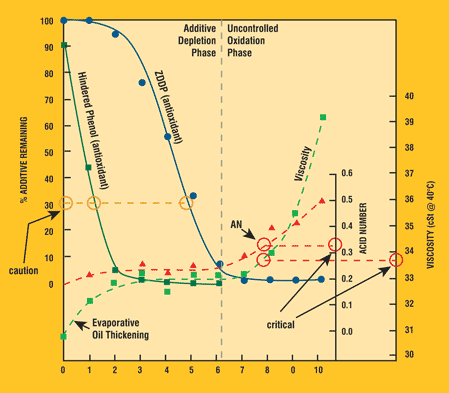
Figure 1. Correlating Changes in RUL to Oxidation Life Cycle8
Objectives of Measuring AN
AN is the measure of acid concentration in a nonaqueous solution. It is determined by the amount of potassium hydroxide (KOH) base required to neutralize the acid in one gram of an oil sample. The standard unit of measure is mg KOH/g. AN does not represent the absolute acid concentration of the oil sample. The AN measurement detects both weak organic acids and strong inorganic acids.
A change in the acid concentration of an oil can originate from multiple sources. Acidic contaminants, wrong oil, alkaline-reserve depletion and oxidation by-products can cause an increase in acid concentration. Table 1 lists common acids that can be detected.
Understanding the extent of additive depletion is key in determining the RUL of an oil. Some additives are weakly acidic and can elevate the oil's initial AN. As the lubricant ages these additives deplete, thereby reducing the acidity created by the additives. The common antiwear additive, zinc dialkyl dithiophosphate (ZDDP), produces certain AN trends during lubricant aging.
Concurrently, the oil is possibly being contaminated with acidic constituents, increasing the acid content in the oil. The combined effects of additive depletion, acidic contamination and other acidic-affecting events create a challenge in determining what the AN represents.
Figure 1 shows the underlying components that affect the AN during lubricant aging. It can be seen that during an induction period the antioxidant additives are depleting; once these additives are depleted, the base oil begins to oxidize if the stressing conditions are sufficiently high. By trending the AN, this increase can be detected.
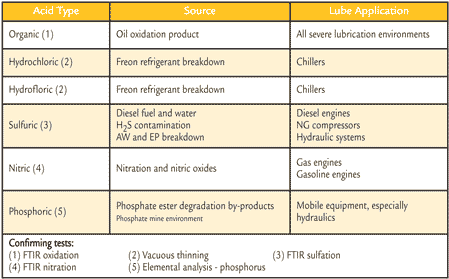
Table 1. AN May Detect These Corrosive Oils
Nomenclature Used in Industry
Total Acid Number vs. Acid Number
Currently in North America, the term total acid number (TAN) is being replaced with acid number (AN). This change is based on the fact that AN tests do not detect the total acid concentration of the lubricant. The acid concentration of the lubricant contains both strong and weak components. Strong acidic components are referred to as SAN.
The weak components and the strong components are typically combined as AN. Even though AN is comprised of both acidic components, it does not represent all acidic components in the lubricant. For instance, the AN and base number (BN) tests are not affected by extremely weak acids and bases that have a dissociation constant of less than 10-9. This is the reason that TAN is being replaced by AN.
pH vs. AN
The pH and AN test methods measure different aspects of the oil's acidic or alkaline character. The pH test method measures the apparent pH of the oil. The apparent pH is a representation of how corrosive the oil may be, but it does not indicate the concentration of acidic or alkaline constituents. The pH test method is useful in applications where corrosive oil could cause considerable damage. It is also valuable in lubricant systems with a high potential for the formation or the contamination of strong acids.
The AN and BN test methods respectively measure the concentration of acidic and alkaline constituents. Both acidic and alkaline constituents can exist in oil at the same time. In fact, some additives are amphoteric, meaning they can behave as either a base or an acid. In some oils, it is important to monitor both the AN and BN to determine the reactions in the oil.
AN and BN do not indicate the strength of the acidic or alkaline constituents in the lubricant, which reduces their ability to indicate the oil's corrosiveness. AN has a better ability than pH to detect and monitor weak acids, which do not readily dissociate in water. This prevents the pH test method from obtaining a good indication of how the weak acid concentration is changing in the lubricant.
Standardized Methods
Table 2 lists the current ASTM standard test methods for determining AN. Each test has been designed for specific purposes, with ASTM D664 and ASTM D974 being the two most commonly used tests. ASTM D1534 and ASTM D3339 are similar versions of D974, used for special cases. AN tests can be broken up into two titration categories: potentiometric or colorimetric.
The potentiometric method uses a potentiometer to detect the acidic constituents and coverts it to an electronic read out. The output is plotted and analyzed to determine the inflection of the test method. The colorimetric method uses paranaphthol-benzene, which responds to a change in the pH indicator that has been added to the solution. Once the acidic constituents have been neutralized by the KOH, the sample will change from orange to blue-green, indicating the end point.
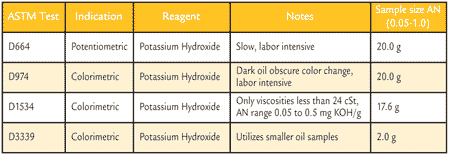
Table 2. Common ASTM AN Test Methods
ASTM AN Tests
ASTM D664 measures acidic constituents by using a potentiometer to determine an end point. This method can be used to measure both AN and SAN. To prepare the sample a mixture of toluene, isopropyl alcohol and water is dissolved into a sample. Potassium hydroxide is then titrated into the solution using a burette. The potentiometer output is monitored while the KOH is titrated into the solution.
If the inflection is indistinguishable, the buffer potential will be considered the AN. The inflection point is commonly used on new oil; however, for used oils the inflection may become indistinguishable requiring the use of the buffer potential as the end point.
ASTM D974 is the measure of acidic constituents using a color change to indicate the inflection. The sample is dissolved into a solution of toluene, p-naphtholbenzne, and isopropyl alcohol containing water. The solution is titrated with KOH while the color is monitored. This test is used on new oils and oils that are not excessively dark.
ASTM D1534 is similar to ASTM D974 in that they both use a color change to indicate the end point. ASTM D1534 is designed for electric insulating oils (transformer oils), where the viscosity will not exceed 24 cSt at 40°C. The standard range of applications is for oils with an AN between 0.05 mg KOH/g and 0.50 mg KOH/g, which is applicable to the transformer oils.
ASTM D3339 is also similar to ASTM D974, but is designed for use on smaller oil samples. ASTM D974 and D664 roughly use a 20 g sample; ASTM D3339 uses a 2.0 g sample, as shown in Table 2.
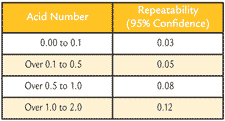
Table 3. D974 Repeatability from ASTM Standard
Modified Tests
AN tests are typically conducted to obtain an accurate indication of additive depletion and possible contamination of ingressed acids. The standard ASTM methods are time consuming, have relatively poor reproducibility and utilize hazardous materials. In an effort to control the source of these issues, many modified versions of the AN test are currently being used. Each test is specific to its application. For example, a lab may automate the test to reduce labor and increase throughput.
For Used Oil Analysis Labs
Laboratories modify tests to improve throughput while decreasing the use of hazardous materials and their cost. Throughput, or speed, is important to larger laboratories because it is necessary to find the fastest test that does not sacrifice quality. Cost also plays a major role. A standard test slate provided by a lab may also include particle count, viscosity at 40°C, etc.
The cost of this standard test slate needs to be affordable for the end user; therefore, each individual test performed may need to be streamlined to ensure both quality and economy are achieved.
For Field Testing
Field test kits are often used as a first-line AN test. They typically contain premeasured reagents that allow for convenient field testing. Some of the field kits use a pass/fail test, which involves adding a preset amount of KOH to the solution. This indicates whether the AN has reached a specific point.
Field tests can also report actual results. For example, one such kit uses a volume-sampling syringe to ensure that the oil samples are the same size. A disposable burette is used to titrate the KOH. Because the oil sample is a specific size, the burette has been scaled to indicate the AN. Once the color has changed, the user only can read the acid number from the burette.

Table 4. D974 Reproducibility from ASTM Standard
Advantages and Disadvantages
Repeatability
ASTM defines repeatability as "the difference between successive test results obtained by the same operator with the same apparatus under constant operating conditions on identical test material". Based on this definition, using D664, data was found to be within +/- 7 percent of the mean 95 percent of the time for fresh oils using the inflection point method or +/- 12 percent of the mean for used oils with the buffer end point method.
ASTM D974 has the repeatability as stated in Table 3. For example, a sample that has a 0.15 AN could vary from 0.10 AN to 0.20 AN for ASTM D974 and could vary 0.17 to 0.13 AN for ASTM D664.
Repeatability can be obtained on a modified test. A good lab should be able to tell how reliable its modified version is. This confirms that comparing results from one single lab or test procedure is best.
Reproducibility
ASTM's definition of reproducibility is "the difference between two single independent results obtained by different operators working in different laboratories on identical test material." Ninety-five percent of the time, the reproducibility of ASTM D664 is +/- 20 percent of the mean for fresh oils using the inflection point method or +/- 44 percent of the mean for used oil using the buffer end point method.
For example, if a mean AN was 0.10 you could expect results from 0.14 to 0.06 95 percent of the time. The reproducibility of ASTM D974 is shown in Table 4. Consider that you received an oil analysis report from multiple labs on the same oil. It has a mean AN of 0.05, and the results could vary from 0.09 to 0.01.
It is hard if not impossible to compare results between labs when modified AN tests are used. Quality labs will likely have a correlation to the ASTM standard; unfortunately, this would also incorporate more error. It is best practice to compare only results from the same test for trending purposes.
According to ASTM, "the AN obtained by this standard (D664) may or may not be numerically the same as that obtained in accordance with test methods D974 and D3339." However, the magnitude of the results should be the same. By trending results from one specific test method, additive depletion and contamination can be detected.
Dos and Don'ts of Comparing Results
Comparing results between samples can become complicated if proper control is not used. There are many aspects which may and normally will affect the results from an AN test. As stated previously, there are multiple test methods used. Some of the methods are within ASTM standards and some are modified. The average AN result from a laboratory will likely be from a modified test method.
Dos
Compare results to historical results on the lubricant (trending).
Verify which lab has analyzed the lubricant and the test used.
Consistently use the same lab and test method for a specific lubricant.
Ensure a representative sample is provided to the lab.
Don'ts
Don't switch back and forth between methods.
Don't switch back and forth between labs. Don't delay oil analysis; instead, provide the sample to the lab as soon as possible.
Don't compare results between different methods.
Common Trends of AN
Trending
In the world of AN tests, there is a current state of disillusionment. Each laboratory provides results from its own modified test methods, which forces the end user to rely on precision over accuracy. First, the user must be wary of comparing results between labs. In an ideal environment, both accuracy and precision would be provided. In a next-to-ideal world, only accuracy would be provided.
Simple mathematics could be used to determine the exact value, but in the real world of AN tests, the precision of each individual labs is what can be counted on. The results are not on the true mark, but relative to each other they are good. Comparing results from different labs would result in values all over the board. By focusing on the precision of one lab or test procedure, a trend emerges. Trending can enable the end user to properly evaluate his/her lubricant with greater confidence.
General Trends
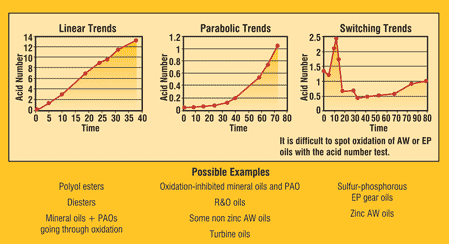
Trending results is the best way to work around the accuracy discrepancies that come from using AN results in machine condition monitoring. By using results from one specific test or lab, the ability to trend is good. Figure 2 illustrates the common trends found in lubricants. Linear trends are for some ester-based synthetics and oils going through oxidation. It represents the linear oxidation of the base oil.
The parabolic curves may characterize rust and oxidized (R&O) oils. The AN remains constant during the additive depletion induction phase. Once the R&O additives have depleted, the base oil will begin to oxidize. The switching trend is representative of EP oils, where some of the additives are acidic. As additives deplete and react, the AN varies. These effects make it hard to trend EP oils unless the normal switching pathway is known in advance.
AN is an important tool in the oil analysis industry when used properly. Understanding how the AN is calculated and what variances exist will help in interpreting the results. SAN is usually not tested, but it may be useful to an oil analysis program if protection from corrosion is important or if there is a possibility of contamination from an inorganic acid.
The two commonly used ASTM test methods both exhibit issues that create the need for modified tests. With the modified tests currently being used in industry, it is important to remember why they are in place and the implications when comparing results. Being able to properly trend results will enable end users to adequately evaluate their oil condition.
References
ASTM D664: Standard Test Method for Acid Number of Petroleum Products by Potentiometric Titration. American Society of Testing and Materials International, West Conshohocken, Pa.
ASTM D974: Standard Test Method for Acid Number and Base Number by Color-Indicator Titration. ASTM Intl., West Conshohocken, Pa.
ASTM D1534: Standard Test Method for Approximate Acidity in Electrical Insulating Liquids by Color-Indicator Titration. ASTM Intl., West Conshohocken, Pa.
ASTM D3339: Standard Test Method for Acid Number of Petroleum Products by Semi-Micro Color Indicator Titration. ASTM Intl., West Conshohocken, Pa.
Finch, Stephen. "Evaluation of New Field Test Methods for Base Number and Acid Number in Lubricating Fluids." Dexsil.
Smart, Clifford L. "Get Smart with Improved TAN Titrations." Practicing Oil Analysis magazine. October 2000.
"Interview Helps Clarify Questions Surrounding AN/BN Test Methods in Used Oil Samples." Practicing Oil Analysis magazine. May 2003.
Kauffman, R.E. "Rapid Determination of Remaining Useful Lubricant Life." Handbook of Lubrication and Tribology, Volume III. E. Richard Booser, Editor. CRC Press, Boca Raton, Fla. 1994.
Snook, Willet A. "Used Engine Oil Analysis." Lubrication, Volume 54, Number 9, 1968.
Ball, Peter G. "New pH Test Offers Benefits over TAN/TBN." Practicing Oil Analysis magazine. September 1998.
Oil Analysis Level I Course Manual, Noria Corporation. 2006.
No comments:
Post a Comment