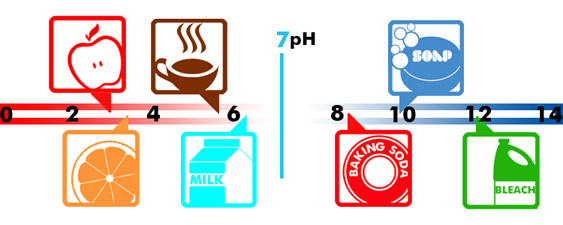
The indicator for acidity, alkalinity, or basic is known as the pH value. A pH value of 7 means a substance is neutral. The lower value indicates acidity, and a higher value is a sign of alkalinity. To better understand the range in pH, take a look at these examples:
- Apple Juice - 3
- Orange Juice - 3.5
- Coffee - 5.5
- Milk - 6.2
- Baking Soda - 8.5
- Soapy water - 10
- Bleach - 12
In addition, many of the foods we eat contain an acidic pH because of their bacteria killing functions.
pH Values of Water Completely Explained
So, what does pH mean for water? Basically, the pH value is a good indicator of whether water is hard or soft. The pH of pure water is 7. In general, water with a pH lower than 7 is considered acidic, and with a pH greater than 7 is considered basic. The normal range for pH in surface water systems is 6.5 to 8.5, and the pH range for groundwater systems is between 6 to 8.5. Alkalinity is a measure of the capacity of the water to resist a change in pH that would tend to make the water more acidic. The measurement of alkalinity and pH is needed to determine the corrosiveness of the water.
In general, water with a pH < 6.5 could be acidic, soft, and corrosive. Acidic water could contain metal ions such as iron, manganese, copper, lead, and zinc. In other words, acidic water contains elevated levels of toxic metals. Acidic water can cause premature damage to metal piping, and have associated aesthetic problems such as a metallic or sour taste. It can also stain laundry and cause "blue-green" color staining on sinks and drains. More importantly, there are health risks associated with these toxins. The primary way to treat the problem of low pH water is with the use of a neutralizer. The neutralizer feeds a solution into the water to prevent the water from reacting with the household plumbing or from contributing to electrolytic corrosion. A typical neutralizing chemical is soda ash. Also known as sodium carbonate, soda ash works to increase the sodium content which increases pH. Water with a pH > 8.5 could indicate that the water is hard. Hard water does not pose a health risk, but can also cause aesthetic problems. These problems include an alkali taste to the water (making that morning coffee taste bitter!), formation of scale deposits on dishes, utensils, and laundry basins, difficulty in getting soaps and detergents to lather, and the formation of insoluble precipitates on clothing.
According to a Wilkes University study, the association of pH with atmospheric gases and temperature is the primary reason why water samples should be tested on a regular basis. The study says that the pH value of the water is not a measure of the strength of the acidic or basic solution, and alone cannot provide a full picture of the characteristics or limitations with the water supply.
While the ideal pH level of drinking water should be between 6-8.5, the human body maintains pH equilibrium on a constant basis and will not be affected by water consumption. For example, our stomachs have a naturally low pH level of 2 which is a beneficial acidity that helps us with food digestion.
No comments:
Post a Comment