Estimating Turbine Oil Oxidation
From steam turbine to gas turbine, from power
generation to refining, turbines are pervasive throughout industry.
While turbine systems can endure a whole host of different failure
modes, studies by major turbine manufacturers such as General Electric
have pointed to the lubricant as one cause of poor reliability.
However, other factors such as maintenance and operational
practices, electrostatic discharge, contamination, and lubricant
chemistry have been identified as root causes. Turbine oils must endure a
host of different challenges due to heat from the process itself,
compressive heating, aeration, and internal and external contamination,
including water and particles. Perhaps the most misunderstood failure modes are those induced by the turbine oil itself. While turbine oils are naturally pure, well-formulated oils, the long-term stress caused by adverse operating conditions can result in both thermal and oxidative degradation of the oil which can cause problems with the reliability and operability of turbine systems.
Even in the most controlled systems, turbine oils are subjected to a number of stressing factors that can lead to premature degradation of the fluid. These include heat, aeration, water and metal catalysts from the machine itself. While the chemical processes are complex, the end result is the same: the formation of by-products of oxidation such as sludge and varnish.

Figure 1. Varnish Formation on a Valve
Sludge and Varnish Sludge and varnish formation is a sequential process. Initially, heat in combination with aeration causes base oil molecules to chemically react with oxygen. This forms soluble by-products including ketones, hydroperoxides and organic acids. Over time, these by-products can combine either physically - a process referred to as agglomeration - or chemically due to further reaction, eventually becoming large enough that they drop out of suspension in the oil, forming solid or semisolid deposits on oil-wetted machines surfaces. Compounding their effect, by-products of oil degradation are often sticky or resinous in nature. This can cause a host of problems including servovalve stiction, buildup on spool metering edges, restriction of oil flow, reduced spool-to-bore clearances, thermal insulation of the valve, combination with other particles and the loss of stick-slip control. Recent research findings1 point to many contributing causes in the oxidation to varnish (Figure 1) process, such as:
- highly localized overheating of the lubricant due to flow restriction or pooling;
- microdieseling which occurs when tiny air bubbles undergo pressure-induced, high-temperature implosions that break down oils;
- static electricity generated by some filter media leading to spark discharges that may subject the oil to localized temperatures above 10,000°C;
- chemical degradation resulting from chemical reactions within a previously used oil which has not been adequately drained/flushed from the system (liquid catalyst);
- chemical degradation from catalyst properties of solid or semisolid varnish or varnish precursors (varnish or precursor sludge catalysts);
- additive chemistries and base oil types used in lubricant formulations that greatly affect the propensity of a finished lubricant to generate varnish.

Figure 2. Ultra-weak Luminescence Detector and Spectrometer
Ultra-weak Chemiluminescence Method Within the fields of food sciences, biotechnology research and basic materials characterization, there are several reliable and repeatable methods for determining the level of oxidation within samples. Ultra-weak chemiluminescence (UWCL) analysis is one such method with a proven track record as a versatile, reliable, accurate and repeatable methodology for studying oxidation of liquids, solids and even gases. Materials as diverse as blood fats, food oils2, beer, pharmaceutical petroleum oils, polymers3,4,5 and even ramen noodles have all been intensively studied and characterized using UWCL. Because of its versatility in characterizing oxidation in these materials, this method was applied to determine if it could be used to successfully measure the early onset of turbine oil oxidation.
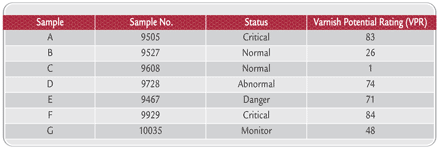
Table 1. Oil Samples
Let There Be Light It is well known that low-level luminescence is naturally produced from many kinds of materials. Luminescence simply refers to any process or material that emits light energy. By definition, chemiluminescence refers to luminescence caused by a chemical reaction, such as the glow of a firefly's tail. Specifically applied to this study of material oxidation, chemiluminescence is induced by heating the sample in question inside a reaction chamber. By heating the sample, unstable molecular species such as hydroperoxides that are intermediates in the oxidative breakdown of organic materials start to decompose. This decomposition liberates an unstable form of oxygen referred to as singlet oxygen or excited carbonyls. As the unstable singlet oxygen or excited carbonyls are liberated, light energy is emitted.
In this study, we tried to estimate the oxidation level of turbine oil by measuring UWCL and measuring the correlation between the UWCL and an established varnish potential indicator test known as quantitative spectrophotometric analysis (QSA®).6
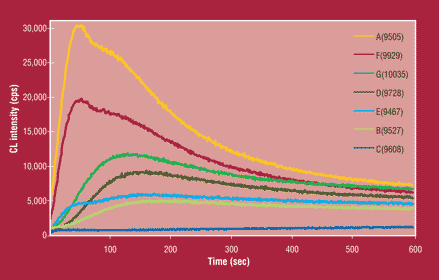
Figure 3. UWCL Time Course Change of Turbine Oil at 130°C in Air
Materials and Method Turbine Oils In the initial study, seven in-service oil samples with the same fully formulated Group II turbine oil were chosen so that the overall chemistry and additives would be consistent. The samples were chosen so that a broad range of QSA® varnish potential ratings (VPR) range would be represented (Table 1). Measurement of Turbine Oil UWCL
To measure the UWCL of the turbine oil samples, a chemiluminescence analyzer model CLA-FS3 (Tohoku Electronic Industrial Company, Sendai, Japan) was used (Figure 2). Each oxidized 2-milliliter sample of turbine oil was placed on a stainless-steel dish (50 millimeters in diameter and 10 mm in height) and the UWCL intensity was measured in air at 130°C for 600 seconds. Result and Discussion
Figure 3 shows the time course change of UWCL of turbine oils at 130°C. There are two peaks at approximately 50 and 150 seconds. The first peak at approximately 50 seconds of exposure to 130°C is due to weaker oxidation bonds that are more easily broken with heat. The underlying chemistry of that oxidation by-product has not yet been fully studied or identified. Although the peak is not as close to QSA® in correlation, it might later prove to have other relationships to varnish formation.
The second peak around 150 seconds of heating presents chemiluminescence due to oxidation by-products which form as singlet oxygen is liberated or excited carbonyls are generated during decomposition at 130°C.
Figure 5 shows the correlation between the integrated UWCL signal between 150 to 154 seconds and the QSA® results. As can be seen, the UWCL intensity correlated well with the QSA® indicator of varnish potential with a correlation coefficient (R2) of 0.765 (authors' footnote), the correlation coefficient represents the degree to which two parameters correlate. It ranges from zero (no correlation) to one, indicating complete correlation. Values in the range of 0.7 and above indicate a high degree of correlation between two observables.
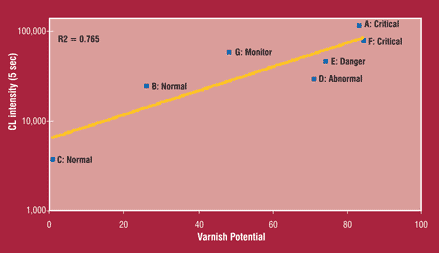
Figure 5. Correlation Between UWCL (150 to 154 seconds) and Varnish Potential Sidebar
This result indicates that the UWCL method can possibly be used to
estimate propensity to form varnish by measuring specific oxidation
compounds of turbine oil. UWCL assay is important for its ability to measure the oxidation levels of either organic or inorganic materials. Likewise, samples can be in solid, liquid or gas states or a combination of states. Sample sizes are small (approximately 2 mL). Test time is less than 20 minutes. Finally, adding reaction-causing chemicals or time-consuming physical preparations are not required.
Although more data on a variety of different lubricant chemistries needs to be collected and testing procedures perfected specifically for turbine oil, it appears from this study that UWCL may be a promising methodology for the rapid and sensitive measurement of turbine oil varnish potential.
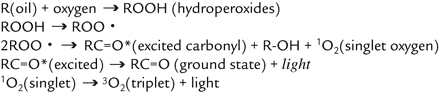
Figure 4. Chemiluminescence Generation Mechanism
When an excited carbonyl species or singlet oxygen is released to the ground state, it gives out its energy as a light. Therefore, this UWCL indicates the amount of hydroperoxides or other oxidized products, and it is possible to measure the degree of oxidation.
These oxidation products seem to correlate with the QSA® predictive index.
Chemiluminescence can be induced by many energies, including heat, light, radiation, chemical reaction or pressure. The key in oxidation characterization is "popping" that oxygen singlet off the compound. When it is liberated, there is a photon emission. The more oxygen liberated at the same stress condition means more light; more light means that more of some specific oxygenated compound is present.
Finally, because multiple oxidation compounds can be measured in the same test, the significance of each compound can be mapped with relation to the goal - predicting varnish potential and measuring prevention or remediation effectiveness.
About the Authors
Mark Okazaki is manager of industrial oils technology for Chevron Products Company.
Rie Yamada is president of Tohoku Electronic Industrial Company Ltd.
Tom Turner is manager of business development for ITA Inc.
References Rie Yamada is president of Tohoku Electronic Industrial Company Ltd.
Tom Turner is manager of business development for ITA Inc.
-
Buddy Atherton. "Discovering the Root Cause of Varnish Formation." Practicing Oil Analysis magazine, March 2007.
-
T. Miyazawa, K. Fujimoto, M. Kinoshita, R. Usuki. J. Am. Oil. Chem. Soc. 1994, 71, 343.
-
N.C. Billingham, D.C. Bott, A.S. Manke. Developments in Polymer Degradation-3; N. Grassie (ed.), Applied Science Publishers, London, 1988, p. 63-100.
-
G.A. George. Luminescent Techniques in Solid-State Polymer Research. L. Zlatkevich (ed.), Marcel Dekker, New York, 1987, p. 93.
-
S.W. Bigger, P.K. Fearon, D.J. Whiteman, T.L. Phease, N.C. Billingham. ACS Div. Polym. Chem., Polym. Prepr., 2001, 42:375-376.
-
Quantitative spectrophotometric analysis is a proprietary varnish potential test performed by Analysts Inc.
-
G. Russell. J. Am. Chem. Soc. 1957, 79, 3817.
No comments:
Post a Comment