Neutralisation of regenerants
Introduction
The quantity of chemicals (expressed in equivalents or g as CaCO3) used to regenerate an ion exchange resin is always equal to the ionic load of the resin plus a certain excess. See the definition of regenerant ratio.In many plants, regulations require that the waste produced by regeneration of ion exchangers is neutral. Mixed regeneration waste is considered neutral when the pH value of the waste is between 6 and 9.
By adjusting the quantity of each regenerant, a neutral regeneration waste can often be obtained. The way to calculate excess acid and excess alkalinity is not straightforward, because Na2CO3 and Na2SiO3 obtained in the spent caustic are also effective to neutralise excess acid. They cause a buffering effect, which eventually provides some flexibility in the adjustment of neutralisation.
| Self-neutralised waste | |
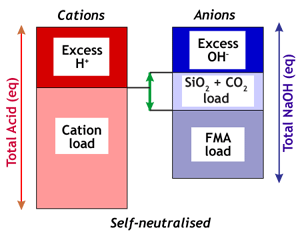 | Spent regenerant is self-neutralised when the excess acid is greater than the excess caustic, but smaller than the excess caustic plus the silica and carbonate load:Ex(H) > Ex(OH) and Ex(H) < Ex(OH) + CO2 + SiO2 The picture on the left shows that not only the excess of NaOH, but also the carbonate and silicate on the resin can neutralise the excess of acid.
When excess acid is between excess caustic and excess caustic plus silica and carbonate load, the IXCalc software I developed for Rohm and Haas shows the same number in the cation and anion excess regenerant fields, namely the excess acid number, which is larger than the strict excess caustic number. You see that easily in the picture on the left.
In other cases, acid or caustic must be added, but only to the limit of the CO2 + SiO2 load as shown in the next two pictures: |
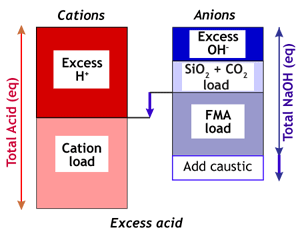
| Excess alkalinity | Excess acidity |
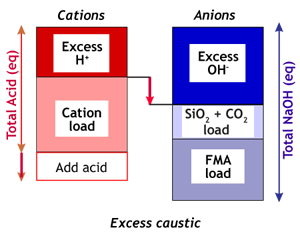 |  |
| This case occurs often when the water treatment system does not include a degasifier. Before neutralisation, the situation is:
Ex(OH) > Ex(H)
In this case, acid must be added until
Ex(H) = Ex(OH)
(or slightly more). | This case occurs often when the water is degassed, and when there is a double stage anion exchanger (WBA + SBA). Before neutralisation, the situation is:
Ex(H) > Ex(OH) + CO2 + SiO2
In this case, caustic must be added until
Ex(OH) + CO2 + SiO2 = Ex(H)
(or slightly more). |
No comments:
Post a Comment