Cation Exchange Capacity (CEC)
CEC, as reported by nearly all soil testing laboratories, is a calculated value that is an estimate of the soils ability to attract, retain, and exchange cation elements. It is reported in millequivalents per 100 grams of soil (meq/100g).
In order for a plant to absorb nutrients, the nutrients must be dissolved. When nutrients are dissolved, they are in a form called "ions". This simply means that they have electrical charges. As an example table salt is sodium chloride (NaCl), when it dissolves it becomes two ions; one of sodium (Na+) and one of chloride (Cl-). The small + and - signs with the Na and the Cl indicate the type of electrical charges associated with these ions. In this example, the sodium has a plus charge and is called a "cation". The chloride has a negative charge is called an "anion". Since, in soil chemistry "opposites attract" and "likes repel", nutrients in the ionic form can be attracted to any opposite charges present in soil.
Soil is made up of many components. A significant percentage of most soil is clay. Organic matter, while a small percentage of most soil is also important for several reasons. Both of these soil fractions have a large number of negative charges on their surface, thus they attract cation elements and contribute to a higher CEC. At the same time, they also repel anion nutrients ("like" charges).
Some important elements with a positive electrical charge in their plant-available form include potassium (K+), ammonium (NH4+), magnesium ( Mg++), calcium (Ca++), zinc (Zn+), manganese (Mn++), iron (Fe++), copper (Cu+) and hydrogen (H+). While hydrogen is not a nutrient, it affects the degree of acidity (pH) of the soil, so it is also important. Some other nutrients have a negative electrical charge in their plant-available form. These are called anions and include nitrate (NO3-), phosphate (H2PO4- and HPO4--), sulfate (SO4-), borate (BO3-), and molybdate (MoO4--). Phosphates are unique among the negatively charged anions, in that they are not mobile in the soil. This is because they are highly reactive, and nearly all of them will combine with other elements or compounds in the soil, other than clay and organic matter. The resulting compounds are not soluble, thus they precipitate out of soil solution. In this state, they are unavailable to plants, and form the phosphorus "reserve" in the soil.
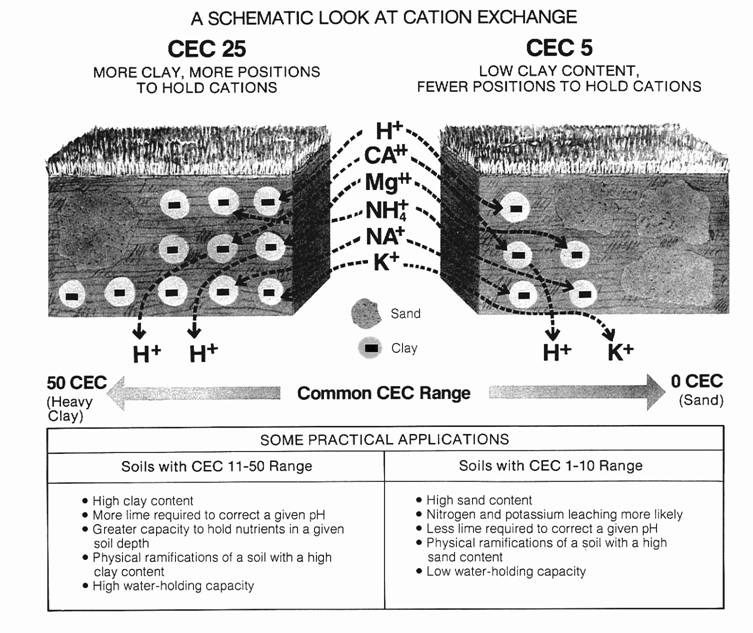
Larger CEC values indicate that a soil has a greater capacity to hold cations. Therefore, it requires higher rates of fertilizer or lime to change a high CEC soil. When a high CEC soil has good test levels, it offers a large nutrient reserve. However, when it is poor, it can take a large amount of fertilizer or lime to correct that soil test. A high CEC soil requires a higher soil cation level, or soil test, to provide adequate crop nutrition. Low CEC soils hold fewer nutrients, and will likely be subject to leaching of mobile "anion" nutrients. These soils may benefit from split applications of several nutrients. The particular CEC of a soil is neither good nor bad, but knowing it is a valuable management tool.
The following, is data on how CEC is calculated at Spectrum Analytic.
Milli-equivalents (Meq.) of Selected Cations and Their Equivalent ppm
| |||||
|---|---|---|---|---|---|
Cation
|
Atomic Weight
|
Valence
|
Milli-equivalents
|
Equivalent
| |
ppm
|
Lbs/acre
| ||||
H+
|
1
|
1
|
1
|
10
|
20
|
Ca++
|
40
|
2
|
20
|
200
|
400
|
Mg++
|
24
|
2
|
12
|
120
|
240
|
K+
|
39
|
1
|
39
|
390
|
780
|
NH4+
|
18
|
1
|
18
|
180
|
360
|
Al+++
|
27
|
3
|
9
|
90
|
180
|
Zn++
|
65
|
2
|
32.5
|
325
|
650
|
Mn++
|
55
|
2
|
27.5
|
275
|
550
|
Fe++
|
56
|
2
|
28
|
280
|
560
|
Cu++
|
64
|
2
|
32
|
320
|
640
|
Na+
|
23
|
1
|
23
|
230
|
460
|
Cation Exchange Capacity (C.E.C.) Calculation
On July 1, 2005, we began to report K, Ca, and Mg in Mehlich 3 ppm as well as our old method of reporting. When we did this we kept the same CEC calculations that we did on our old reports. Therefore, if you are getting a report that has Mehlich 3 ppm K, Ca and Mg reported in ppm you will need to use the following formulas to recalculate to our old converted reporting numbers. Below you will find the formulas.
Lbs K = (M3 K ppm × 0.84) × 2
Lbs Ca = (M3 Ca ppm × 0.75) × 2
Lbs Mg = (M3 Mg ppm × 0.88) × 2
METHOD 1: Use if a buffer pH (BpH) is available.
C.E.C. = (lb K ÷ 780) + (lb Mg ÷ 240) + (lb Ca ÷ 400) + [12 × (7 - BpH)]*
* If buffer pH is 7.0 or greater, use a 0 value as the remainder...Example: (7.0 - 7.1) = 0
METHOD 2: Use if Buffer pH is not available.
C.E.C. = [(lb K ÷ 780) + (lb Mg ÷ 240) + (lb Ca ÷ 400)] × Factor
Multiplication factors to use in method 2
If pH is
|
Use Factor*
|
|---|---|
7.3 or higher
|
1.00
|
7.2
|
1.05
|
7.1
|
1.10
|
7.0
|
1.15
|
6.9
|
1.17
|
6.8
|
1.20
|
6.7
|
1.22
|
6.6
|
1.25
|
6.5
|
1.28
|
6.4 or less
|
Use Method I
|
* The multiplication factor accounts for other cations
Percent Saturation
Both Percent Nutrient Saturation and Percent Base Saturation refer to a measurement, or estimate of the percent of the soil CEC that is occupied by a particular nutrient (nutrient saturation), or the sum of a group of nutrients (base saturation). This information gives us another tool to use in predicting the soils ability to provide adequate crop nutrients, and indicate needed changes in fertilizer or lime programs. A simplified example of percent saturation would be where a soil is capable of holding 100 cations and these 100 "exchange sites" are occupied by the following nutrients.
Nutrient
|
Nutrient Quantity (meq)
|
Nutrient Saturation
|
Base Saturation
|
|---|---|---|---|
Calcium (Ca++ )
|
67
|
67%
|
Sum of nutrient saturation of Ca, Mg, and K
67 + 15 + 3 = 85%
|
Magnesium (Mg++ )
|
15
|
15%
| |
Potassium (K+ )
|
3
|
3%
| |
Hydrogen (H+ )
|
12
|
12%
| |
Others*
|
3
|
3%
| |
Total
|
100
|
100%
|
*Includes Iron (Fe++), Manganese (Mn++), Copper (Cu++), Zinc (Zn++), Sodium (Na+), Aluminum (Al+++), and others.
The percent Nutrient Saturation is the saturation of the individual elements. The percent Base Saturation is the combined percent saturation of the three major cations that have a basic or alkaline reaction (K+, Ca++, and Mg++).
Since a soil test report is typically not measuring and reporting all of the cations that are in the soil, it is common for the sum of the measured cations to add up to less than 100%. Also, when the soil pH is above about pH 7.2, the sum of the cation saturation's may add up to more than 100%. This is because there is likely to be "free" Ca, Mg, and/or Na (unattached to the soil exchange complex) in the soil that is unavoidably extracted by the soil testing process.
Optimum Percent Saturation Ranges
There is some disagreement among agronomists about the value of using "optimum" percent saturation ranges of soil cation nutrients. One school of thought holds that it is very important that the soil contain a specific saturation, or ratio of saturations for each of the major cation nutrients (Ca, Mg, and K). Practitioners of this approach will make recommendations designed to adjust the soil to specific saturation levels. The opposing view is that there can be a wide range of saturation for each of these major cations, with no significant benefit to having particular saturation levels or ratio of saturation levels. The evidence suggests that the primary need is for an adequate amount of each nutrient, regardless of the resulting percent saturation, and that the desired saturation range can be quite broad. To the degree that there is an "ideal" percent saturation range or ratio of cation nutrients, it would be affected by several other factors such as any unique characteristics of a plant species, the intended use of the plants, the nature of the soil itself, and others. Our experience suggests that both the "pounds per acre" and the "percent saturation" philosophies have some merit in different situations, and that both should play a role in making recommendations.
Given that targets for percent saturation's can have some flexibility, the following table lists some suggested saturation ranges that would likely be considered acceptable by most agronomists.
Soil CEC
|
% K
|
% Ca
|
% Mg
|
|---|---|---|---|
0-5
|
4-6
|
50-70
|
10-20
|
6-10
|
3-5
|
50-70
|
8-20
|
11-15
|
3-4
|
50-70
|
8-20
|
16-20
|
2-4
|
50-70
|
8-20
|
21-25
|
2-4
|
50-70
|
8-20
|
26-30
|
1.5-3
|
50-70
|
5-20
|
30+
|
1.5-3
|
50-70
|
5-20
|
Keep in mind that when the soil CEC is between 0 and about 3, the percent saturation has less meaning agronomically. This is because the holding power of the soil is so low that even a deficient amount of a cation nutrient could result in a relatively high saturation. In those cases, the soil test is telling us that we should consider making multiple split applications of those cations needed in large amounts, because the soil in unable to retain any significant amount from a single application. One analogy that seems to illustrate how percent saturation works is comparing it to an irrigation system. The amount of the nutrient is similar to the amount of water applied in irrigation, while the percent saturation is similar to the water pressure of the irrigation system. The amount of water is most critical, but the water pressure plays an important role.
Soil pH and Buffer pH
Soil pH This is a measure of the soil acidity or alkalinity and is sometimes called the soil "water" pH. This is because it is a measure of the pH of the soil solution, which is considered the active pH that affects plant growth. Soil pH is the foundation of essentially all soil chemistry and nutrient reaction and should be the first consideration when evaluating a soil test. The total range of the pH scale is from 0 to 14. Values below the mid-point (pH 7.0) are acidic and those above pH 7.0 are alkaline. A soil pH of 7.0 is considered to be neutral. Most plants perform best in a soil that is slightly acid to neutral (pH 6.0 to 7.0). Some plants like blueberries require the soil to be more acid (pH 4.5 to 5.5), and others, like alfalfa will tolerate a slightly alkaline soil (pH 7.0-7.5).
The soil pH scale is logarithmic, meaning that each whole number is a factor of 10 larger or smaller than the ones next to it. For example if a soil has a pH of 6.5 and this pH is lowered to pH 5.5, the acid content of that soil is increased 10-fold. If the pH is lowered further to pH 4.5, the acid content becomes 100 times greater than at pH 6.5. The logarithmic nature of the pH scale means that small changes in a soil pH can have large effects on nutrient availability and plant growth.
Buffer pH (BpH) This is a value that is generated in the laboratory, it is not an existing feature of the soil. Laboratories perform this test in order to develop lime recommendations, and it actually has no other practical value.
In basic terms, the BpH is the resulting sample pH after the laboratory has added a liming material. In this test, the laboratory adds a chemical mixture called a buffering solution. This solution functions like extremely fast-acting lime. Each soil sample receives the same amount of buffering solution; therefore the resulting pH is different for each sample. To determine a lime recommendation, the laboratory looks at the difference between the original soil pH and the ending pH after the buffering solution has reacted with the soil. If the difference between the two pH measurements is large, it means that the soil pH is easily changed, and a low rate of lime will suffice. If the soil pH changes only a little after the buffering solution has reacted, it means that the soil pH is difficult to change and a larger lime addition is needed to reach the desired pH for the crop.
The reasons that a soil may require differing amounts of lime to change the soil pH relates to the soil CEC and the "reserve" acidity that is contained by the soil. Soil acidity is controlled by the amount of hydrogen (H+) and aluminum (Al+++) that is either contained in, or generated by the soil and soil components. Soils with a high CEC have a greater capacity to contain or generate these sources of acidity. Therefore, at a given soil pH, a soil with a higher CEC (thus a lower buffer pH) will normally require more lime to reach a given target pH than a soil with a lower CEC.
No comments:
Post a Comment