Potassium (K)
Introduction
Potassium is represented by the letter K, which is the first letter of Kalium, the Latin word for potassium. The term “potash” is often used to refer to potassium chloride (KCl), a common potassium fertilizer. The word potash is derived from “pot ashes” which refers to the practice of using the leachate of wood ashes as a source of potassium. The potassium in fertilizer is typically listed as K2O, an oxide form of K. While K2O actually is not present in fertilizers and is not utilized by plants, the term has become the accepted way of designating the amount of K in fertilizers. To convert between K and K2O, simply multiply K × 1.2 to get K2O or divide K2O by 1.2 to get K.
Most readers may be familiar with how fertilizers are labeled. However, for the rest, the following should help to explain the numbering system. When multi-nutrient fertilizer products are labeled with the nutrient analysis (in percentages by weight) the first number is the percentage of N in the fertilizer, the second is the percentage of P2O5, and the third is the percentage of K2O. For example a fertilizer with an analysis of 27-18-10 would contain 27% N, 18% P2O5, and 10% K2O. The terms P2O5 and K2O are used to indicate that these elements are in fertilizer forms rather than pure P or K, or some other form.
Functions of K in the Plant
Potassium does not form a structural part of any plant component or compound. It is required for various metabolic activities and physiological functions. Some of them include the following.
- Role in photosynthesis and plant food formation.
- Role in sugar and carbohydrate production, transport, and storage. A common effect of this K function is an N shortage in legumes when they are short of K. The reason being that the K deficient plants produce and transport less sugar to the legume nodules, thus causing the N-fixing bacteria in the nodules to reduce the amount of N produced.
- Important, in conjunction with Ca and B, in the proper development of cell walls.
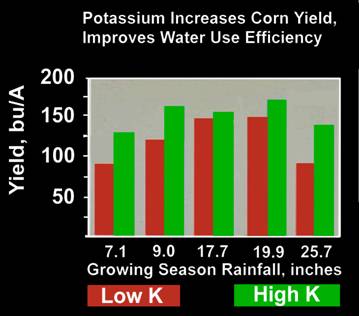
- Controls plant cell turgor and through this the opening and closing of leaf stoma. This in turn controls the plants ability to effectively respond to drought stress.
- Improves a plants ability to combat disease, and to a lesser extent insect damage. Various authorities, reviewing the interaction of K nutrition and plant pests across a wide variety of crop species found the following benefits of proper K nutrition.
| Pathogen | Yield/Growth Increase From K |
|---|---|
| Fungus | 48% |
| Bacteria | 70% |
| Virus | 99% |
| Nematodes | 115% |
| Insects, Mites | 14% |
* Potassium affects various quality factors of fruit and vegetables, such as taste and color.
Crops Utilize Large Amounts of Potassium
| Potassium Utilization | ||||
|---|---|---|---|---|
| Crop | Yield | Harvested Portion | Residue | Total Uptake |
| lb/a | ||||
| Alfalfa | 8 Ton | 480 | 0 | 480 |
| Coastal Bermuda | 8 ton | 400 | 0 | 400 |
| Corn (grain) | 150 Bu. | 44 | 145 | 189 |
| Corn (silage) | 20 Ton | 166 | 0 | 166 |
| Cotton (lint) | 3 bales | 42 | 108 | 150 |
| Fescue | 5 ton | 264 | 0 | 264 |
| Peppers | 14 Ton | 122 | 139 | 261 |
| Potatoes | 25 Ton | 298 | 131 | 429 |
| Rice | 7,000 lb. | 24 | 242 | 266 |
| Sorghum (grain) | 6,000 lb. | 23 | 101 | 124 |
| Soybeans | 60 Bu. | 84 | 205 | 289 |
| Tomatoes | 50 ton | 366 | 150 | 516 |
| Wheat | 80 Bu. | 27 | 134 | 161 |
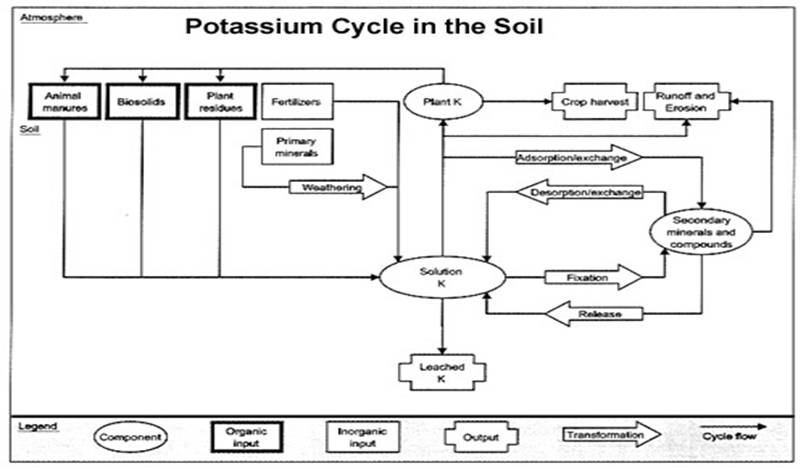
Potassium in the Soil
Soil K content varies widely. However, it is not uncommon for a soil to contain about 20 tons of K per acre (6.67 inches deep). However, very little of this K is available to plants. Plant-available K is typically only 0.1% to 2.0% of the total soil K (40 to 800 lb./acre). Much of the unavailable K is a structural part of various soil minerals.
Factors Affecting K Availability
Soil CEC: Plant-available soil K is in the ionic (electrically charged) form. This charge is positive, making K a cation, represented as K+. Cations are attracted to, and held by negatively charged colloids (primarily clay and organic matter) that make up the cation exchange capacity (CEC) of the soil. The larger the CEC, the more K that can be held by the soil and the higher the soil test needed to adequately feed plants.
Soil test K: Higher soil test K increases the available K, by increasing the amount and balance of K relative to other cations.
Cation Balance: Where there is a significant imbalance between available K and the other major cations (Primarily Calcium, Magnesium, and sometimes Hydrogen, Aluminum, or Sodium), it may affect the availability of K to the crop.
Soil Moisture: K is transported within the soil and is absorbed by plant roots in the soil water. Therefore a water deficiency results in less K absorption.
Soil pH: As the soil pH is reduced (increasing soil acidity) the availability of K is often reduced.
Soil Temperature: Cold soils often reduce the availability of K.
Soil compaction: Compacted soils often reduce the availability of K.
Soil Drainage/Aeration: As soil drainage is improved, K uptake typically improves.
Soil Salinity: Saline soils often have excess sodium (Na). One of the negative effects of excess Na is that it reduces the availability of K.
Interactions
K/Mg ratio: Each of K or Mg can reduce the uptake of the other when the “normal” soil balance does not exist. Typically, we find high K levels inhibiting the uptake of Mg. However, some Midwest soils have enough Mg to reduce K availability, especially to high-demand crops.
Other Cation ratios: There are occasions when K uptake might be restricted due to an imbalance with other cation elements in the soil. For example, in many high pH soils there is an excess of Ca. Competition from this Ca could reduce uptake of K. Strongly acid soils will often have an excess of hydrogen (H), aluminum (Al), iron (Fe), and possibly other cation elements. These excess elements can compete with K for entry into the plant, and/or set up soil conditions that are unfavorable to efficient K utilization.
Soil pH: This subject is intertwined with both of the previous points. While we don't think of K as leachable, in acid soils with low CEC's, we find that K can be leached somewhat. Where initial soil tests or fertilizer programs are not sufficient to offset this loss mechanism, we can see lower yields and crop quality.
Balances and Ratios
For many years, there have been a few people who claim that there is an “Ideal” ratio of the three principal soil cation nutrients (K, Ca, and Mg). This concept probably originated from New Jersey work by Bear in 1945 that projected an ideal soil as one that had the following saturations of exchangeable cations 65% Ca, 10% Mg, 5% K, and 20% H. The cation ratios resulting from these idealizes concentrations are a Ca:Mg of 6.5:1, Ca:K of 13:1, and Mg:K of 2:1.
It is generally accepted that there are some preferred general relationships and balances between soil nutrients. There is also a significant amount of work indicating that excesses and shortages of some nutrients will affect the uptake of other nutrients. However, no reliable research has indicated that there is any particular soil ratio of K, Ca, and Mg that is uniquely superior to another ratio.
Over many years of plot research, cooperative research projects, and a large number of plant analysis samples, we have found that the following ratios are significant much of the time.
- High soil K:Mg ratios (lb./acre or ppm): Over many years of looking at plant analysis samples, we have seen that where the soil test ratio (lb:lb) of K:Mg is greater than 1.5:1; many crops are likely to suffer Mg shortages. This is often in spite of the soil having “adequate” amounts of Mg in the soil. Where the soil test ratio of K:Mg is between 1:1 and 1.5:1, grass crops, including corn may be at risk of an Mg shortage. While such an induced Mg deficiency can reduce yields, it is possibly a bigger problem for livestock that consume the green chop or silage. When these high soil K:Mg ratios are combined with low to marginally sufficient Mg and/or acid soils, the probability of Mg deficiency increases.
High soil Mg can reduce K uptake, but it seems to occur only when the soil Mg saturation is in the range of 25% to 30%, or higher. We have not identified a particular K:Mg ratio that is significant in causing Mg to reduce K uptake.
- Soil K Ratio with the Combined Amount of Ca and Mg: Numerous studies have looked at the possibility of using the K/(Ca + Mg) ratio to explain certain nutrition problems. While this ratio may be useful at some times, often there is no relationship between these cation ratios and crop performance. This is the case whether using the amount of nutrients present (lb/ac or ppm), or the nutrient saturations.
Deficiency Symptoms
The classic and almost universal leaf deficiency symptom is marginal chlorosis of the older plant leaves. However, yield losses typically occur before these symptoms are visible. For example, a crop with insufficient K is likely to wilt sooner in a dry spell. Also, insufficient K could express itself by causing the plants to suffer from more, or more severe disease problems. It might also show as a fruit crop that doesn't quite develop the proper quality or flavor. Possibly the most common and least understood symptom of K shortage is seen as N deficiency in soybeans. When soybeans suffer a K shortage, the plants produce fewer sugars, and have trouble transporting the limited amounts of sugar from the leaves to the roots. The nodulating bacteria depend on this sugar and when it is deficient, they produce less N for the soybean plant to use. All of these are symptoms of K shortages.
High Response Crops
Since K is a major nutrient, all crops require large amounts of K for proper growth and yield. Therefore, it could be said that all crops are “high response”. However, there are some crops that either require higher amounts of K, or are more responsive to proper amounts of K. Much of the K in any plant is in the foliage and other above-ground portions. Therefore, when crops are harvested for leaves and stems, much K can be removed from the field. Seeds contain very little K, so crops grown only for grain typically have relatively low K removal. The fruit of many fruit and vegetable crops often contain relatively high amounts of K, so harvest removes that K from the field. Some crops that can be considered high response or high demand include the following (this list is not all inclusive).
- Alfalfa, apples, bananas, beans (Phaseolus), Bermudagrass, celery, citrus, clover, corn silage, cotton, grapes, Palm Trees/Crops, pasture/forage grasses, peaches, pears, peppers, pineapple, potatoes, pumpkins/squash, rice, small grain forage/silage, sorghum silage, soybeans, sugar beets, sugar cane, sweet potatoes, tobacco, tomatoes, vetch
Toxicity
There is no evidence that K has a direct elemental toxicity. Excess K is more likely to be experienced first as an induced Mg deficiency. Next on the scale of probable high K damage signs might be induced Ca deficiencies. However, excess K may also show up as damage from excess salts. Be sure to calculate the amount of salts that are being applied with row starter fertilizer. It is very unlikely that a commercial crop producer will ever apply enough K to cause salt damage from K alone. However, high rates of K applied to an already salty soil could increase crop damage from the combined effect of all of the salts in the soil. Foliar applications of K can rather easily damage leaves due to simple salt burn.
Use of Potassium in a Fertility Program
In addition to the K uptake and removal, an effective fertilizer program must account for the K supplying or fixation power of the soil. Soil tests are the only effective tool for this job. However, soil testing is not perfect, so producers who want to develop the optimum fertilizer must use plant analysis to determine the actual effect of the fertilizer program.
Increasing Soil Test Potassium
When a soil tests below optimum in K, some of any applied K fertilizer will be tied-up with the colloidal (CEC) complex and other potential fixation mechanisms. To effectively supply the needed K to plants, the grower must apply enough excess K to satisfy the “fixation” power of soils with inadequate K. There are no simple and universal formulas to use in predicting the fixation power in all of the different soils. However, it is a good bet that it will require between 4 and 8 lbs. of excess K2O per acre to increase soil test K 1 lb/acre if the original soil K test is significantly low.
Recommended Rates of K2O
Recommended amounts of K2O are highly variable and depend on the initial soil test, the crop to be grown, and the requested yield goal. The annual recommendation for various crops can range from 0 to almost 1,000 lb K2O/acre. However, we do caution not to apply more than 400 lb K2O/acre in any single application. And, if 300 to 400 lb of K2O/acre is applied in a single application, it is best to incorporate that fertilizer with tillage.
| Sources of Potassium | ||
|---|---|---|
| Material | Chemical Symbol | Typical K2O Analysis |
| Potassium Chloride/Potash | KCl | 60-62% |
| Potassium Sulfate | K2SO4 | 50-53% |
| Potassium-Magnesium Sulfate | K2SO4 • 2MgSO4 | 22% |
| Potassium Nitrate | KNO3 | 44-46% |
| Manure | (not applicable) | 0.4-1.0% |
| Potassium from Manure 1) | ||
|---|---|---|
| Animal Type | K2O (lb/ton) | |
| From | To | |
| Dairy | 6.3 | 9.5 |
| Beef | 7.6 | 11.4 |
| Veal | 7.2 | 10.8 |
| Swine | 8.6 | 13.0 |
| Sheep | 15.6 | 23.4 |
| Goat | 12.1 | 18.1 |
| Layers | 10.6 | 15.8 |
| Broilers | 10.0 | 15.0 |
| Turkey | 13.5 | 20.3 |
| Horse | 7.2 | 10.8 |
No comments:
Post a Comment