Nitrogen (N)
The purpose of this paper is to give the reader a brief overview of some of the major points related to using nitrogen fertilizer materials. Most people working in agriculture and other plant management industries should search out more detailed information on the subject. Such information is readily available from Spectrum Analytic, and many other sources.
Function of N in plants
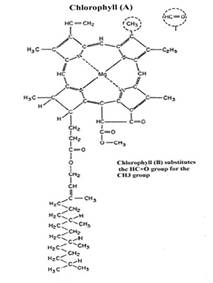 |
Nitrogen is a structural component of several essential plant parts and compounds. They include…
- N is a structural component of chlorophyll
- N is a structural component of the nucleic acids (DNA, RNA) in each cell
- N is a structural component of all proteins
As a result of these functions, corrections of N shortages result in large gains in vegetative growth, much higher protein levels, and much higher yields of grain, fruit, and vegetative plant organs. While these gains are normally desirable, excess amounts of N, either in absolute terms or sometimes in the ratio of N to other elements, can have a negative impact on some aspects of various yield components.
Crops Utilize Large Amounts of N
| Nitrogen | ||||
|---|---|---|---|---|
| Crop | Yield | Harvested Portion | Residue | Total Uptake |
| lb/a | ||||
| Alfalfa* | 8 Ton | 448 | 0 | 448 |
| Coastal Bermuda | 8 ton | 400 | 0 | 400 |
| Corn (grain) | 175 Bu | 131 | 100 | 231 |
| Corn (silage) | 20 Ton | 166 | 0 | 166 |
| Cotton (lint) | 3 bales | 93 | 87 | 180 |
| Fescue | 5 ton | 190 | 0 | 190 |
| Peppers | 14 Ton | 68 | 93 | 161 |
| Potatoes | 25 Ton | 174 | 94 | 268 |
| Rice | 7,000 lb. | 85 | 27 | 112 |
| Sorghum (grain) | 6,000 lb | 90 | 88 | 178 |
| Soybeans* | 60 Bu | 240 | 75 | 315 |
| Tomatoes | 50 ton | 266 | 107 | 373 |
| Wheat | 80 Bu | 92 | 58 | 150 |
| *Legumes get most of their N from the atmosphere | ||||
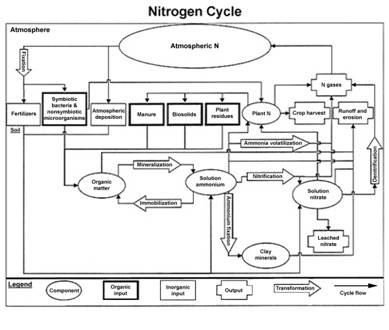
Nitrogen in the Soil
Nitrogen has a complicated cycle. Some features unique to N are that N does not accumulate in the soil to any significant degree. Nitrogen is highly subject to losses from the soil, yet there is a large reservoir of N in the atmosphere. Of course, most plants (non-legumes) are not able to directly utilize atmospheric N.
Plants take up N in several forms. These include nitrate-N (NO3-N), ammonium-N (NH4-N), and urea (CO[NH2]2). Other forms of N must be transformed to one of these forms before plants can utilize the N.
The N in organic residues exists primarily in large organic molecules which cannot be taken into plant roots. These N sources must be decomposed and transformed into one of the previously mentioned available forms of N. Therefore, a significant portion of “organic” N is not immediately available to plants.
Factors Affecting Availability
The principle factors affecting N availability to a crop are
- The amount N applied
- Excess soil moisture
- Residual soil N from previous crops or conditions
- Organic N available from applied manure, municipal sludge, or other residues.
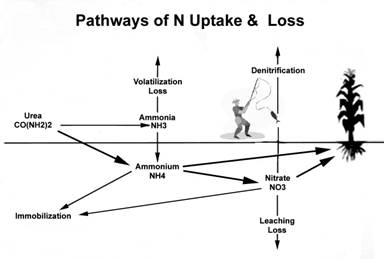
Nitrogen loss after application has been a major concern for years. Nearly all significant pathways of N loss are the result of excess moisture. As it happens, the most loss-prone form of N is nitrate-N (NO3-N).
N-Loss Pathways and Mechanisms
In the early 80's agronomists at Agrico Chemical Co. reviewed a range of information and compiled the following table on the “typical” fate of fertilizer nitrogen after application. While the data may not be precisely accurate in all situations and does not represent the most extreme possibilities, it appears to put the different loss pathways into a relative context.
| Fate of Fertilizer Nitrogen after Application | ||
|---|---|---|
| Crop Uptake* = 40% to 70% of Applied N | ||
| Loss Mechanism | Form Affected | Potential Loss |
| Immobilization | NH4, NO3 | 10-40% |
| Erosion | NH4 | 0-20% |
| Denitrification | NO3 | 5-35% |
| Leaching | NO3 | 0-20% |
| NH3 Volatilization | Urea | 0-30% |
| * Some or much of the missing portion of the applied N (30% to 60%) May be retained in the field and become available to succeeding crops | ||
Immobilization is the process whereby soil organisms consume inorganic N sources in order to grow and reproduce. Most of these organisms are bacteria and other microorganisms which have short life spans. As they die, they contribute to the soil organic matter and the N trapped in their bodies is slowly recycled back to mineral N forms, becoming available to crops and other higher plants. While this process produces many benefits to soil and crops, the N initially consumed is temporarily unavailable to crops. Immobilization is not considered a major source of N loss to the current crop and typically does not involve significant amounts of fertilizer N. Residues and soil additives with high carbon to nitrogen ratios (C:N) increases the amount of N immobilized while C:N ratios around 20 or lower result in N mineralization (N release).
| Some Examples of C:N ratios of Common Materials are as Follows | ||
|---|---|---|
| Group | Type | C:N Ratio |
| Humus | Natural | 10 |
| Manure | Rotted, General | 20 |
| Crop Residue | Corn | 60 |
| Wheat | 80 | |
| Rye Straw | 350 | |
| Sugar cane | 50 | |
| Green Manure | Sweet clover | 12 |
| Red clover | 23 | |
| Green Rye | 36 | |
| Sawdust | various | 350 - 999 |
Erosion is the loss of soil, and whatever nutrients are attached to the soil, from the field. Since NH4-N is a cation (positive charge) that is attached to the CEC complex of the soil, it will be lost with any soil that is lost. This loss mechanism is not considered a significant problem in most fields since the adoption of tillage practices that have greatly reduced erosion in most fields.
Denitrification has been said to be the major mechanism of N loss in Illinois and Ohio, and it is probably the major cause of significant N loss in most crop producing areas. Denitrification occurs when soils are saturated during relatively warm conditions. Soil saturation causes a shortage of gaseous oxygen (O2) in the soil atmosphere. Some soil organisms that utilize gaseous O2 in the soil can also utilize other forms of oxygen. When the soil is saturated, these organism can remove the needed oxygen from nitrate (NO3), converting it to gaseous N (N2). Once the N is in the gaseous form, it is unavailable to plants, other than legumes. The N2 ultimately escapes to the atmosphere. One point of confusion is that some people believe that they must see standing water on the soil surface before significant N losses can occur. This is not necessarily so. If the top few inches of soil is unsaturated and the deeper soil is saturated, then the deeper soil can have significant N losses. Since much of the N may be in the 6” to 12” soil depth, saturation of this soil zone can result in considerable N loss.
| Rates of Denitrification in Saturated Soils | ||
|---|---|---|
| Soil Temperature | Days Saturated | Loss of applied N |
| 55 - 60° F | 5 | 10% |
| 10 | 25% | |
| 75 - 80° F | 3 | 60% |
| 5 | 75% | |
| 7 | 85% | |
| 9 | 95% | |
Leaching is the process whereby nitrate nitrogen (NO3-N) is moved downward in the soil profile as water is moving down through the soil profile. Leaching of NO3-N is possible because NO3 is an anion (negative charge) and is repelled from most soil particles. This keeps it in the soil solution, unattached to the soil CEC complex and subject to moving in whatever direction the soil water moves. As might be expected, leaching is more of a concern in sandy soils, since water moves more freely through them. Nitrate that is leached below the root zone is not available to the crop. However, keep in mind that as the soil dries out, some of the water deeper in the soil is drawn toward the surface. That water may have significant amounts of NO3-N in it and this N can become available to the crop later in the season. Things begin to get very complicated when we try to predict N losses from leaching due to the influence of factors such as differing soil texture at various soil depths, the effects of intermittent rainfall of various amounts broken by dry periods of various lengths and temperatures, the effect of tile lines, and a host of other variables.
Volatilization is the process whereby soil N forms are converted to ammonia (NH3) gas. If the NH3 is formed at the soil surface, there is the opportunity for N loss to the atmosphere. Keep in mind that volatilization is, for all practical purposes, limited to surface applied N sources. This process is most commonly associated with surface applied urea. This is because urea undergoes a unique process as it is converted to NH4 and ultimately NO3. Without getting into the precise chemical reactions of urea, we can say that an intermediate product in the process is NH3. When this NH3 is formed at the soil surface, it is subject to potential escape to the atmosphere. If, on the other hand, the urea is under the soil surface when the NH3 is formed, it quickly combines with water to form NH4 and is retained on the CEC complex of the soil. The soil pH, and more importantly the pH of the soil immediately around the fertilizer granule or droplet can have a significant effect on N losses. High soil pH around the fertilizer leads to higher losses. Volatilization losses are normally insignificant if the nitrogen fertilizer is lightly incorporated into the soil. As can be seen in the following table, while urea is the most significantly affected N fertilizer, there can be some N loss from other forms as well.
| Volatile Loss of NH3 from Surface Applied N (% N Loss in 14 days) | ||
|---|---|---|
| Fertilizer | No Lime | Limed |
| Ammonium sulfate | 0.4 | 19.7 |
| Urea | 29 | 36 |
| Ammonium nitrate | 0.3 | 3.4 |
| Leon fine sand, pH 5.8 | ||
Interactions of N with Other Elements
NH4 and P Perhaps the best documented interaction of N with other nutrients is the relationship between ammonium-N and P uptake. Early research showed that when NH4-N was closely associated with P in a fertilizer source, the plants took up more P. When NO3-N was used, or when the NH4-N was separated from the P source, the P uptake was reduced.
Other than the previously mentioned NH4-P interaction, N does not have other similar close interactions with other nutrients. However, N shortages can dramatically reduce the uptake of most other elements. This appears to be the simple result of a plants loss of vigor, and perhaps lower demand for other nutrients with an N shortage. From our many years of doing plant analysis interpretations, it appears that an N shortage most dramatically reduces the uptake of Mg and Cu. However, our experience supports the previous statement that all other nutrients are typically affected as well.
Balances and Ratios
The primary concern of growers in this area should be to avoid excess N in relation to other nutrients. Many times we hear that “high” N rates are detrimental to crop quality (not to mention the environment). In many of these cases, the supposedly high N rate would not have been detrimental if it was balanced with proportionately strong amounts of other nutrients, especially K. Plants having high N uptake without proportional amounts of at least a few other nutrients can be more subject to disease infection and greater physical damage from insects or environmental factors. Crops that receive high rates of N fertilizer, which also have a shortage of one or more other nutrients, are not likely to properly utilize all of the applied N. This can lead to excess N in the soil or water, or excess nitrates in forage or other crops.
Plant Deficiency Symptoms
Typical N deficiency symptoms are a general chlorosis of the older leaves on a plant along with slower growth and generally smaller plants. Some plants have more specific visual symptoms. Corn, for example will have V-shaped chlorotic tissue extending from the tips of the older leaves toward the stalk, in addition to the other mentioned symptoms.
Legumes can also suffer from N deficiency, even though they are capable of making mineral N in the associated N-fixing nodules. There can be several reasons for N shortages in legumes.
- Some legumes like edible beans simply don't produce enough N in healthy nodules
- Legumes that are not inoculated, and are planted on land that does not contain a resident population of the correct rhizobia will not produce the nodules required for N production.
- A shortage of other nutrients or other soil condition may prevent adequate N production. For example, soybeans suffering from a K shortage are likely to suffer from N deficiency as well, because the nodules will be deprived of the sugars needed for healthy rhizobia and adequate N production.
Using Nitrogen in a Fertility Program
It is important that growers manage N properly to gain maximum efficiency and profits from its use. This paper is much too short to effectively cover all aspects of efficient N management in most crops and conditions. Instead the reader is referred to the many books and Extension publications on the subject. There is also much information about N management on the internet.
| Common Nitrogen Sources | |
|---|---|
| Ammonium Sources | Percent N |
| Anhydrous Ammonia | 82 |
| Aqua Ammonia/N Solutions | 21-49 |
| Ammonium Nitrate | 33.5-34.0 |
| Ammonium Nitrate-Sulfate | 26 |
| Ammonium Nitrate/Lime | 20.5 |
| Ammonium Sulfate | 21 |
| Monoammonium Phosphate (MAP) | 11 |
| Diammonium Phosphate (DAP) | 18-21 |
| Ammonium Chloride | 26 |
| Urea | 46 |
| Nitrate Sources | Percent N |
| Sodium Nitrate | 16 |
| Potassium Nitrate | 13 |
| Calcium Nitrate | 15.5 |
| Slowly Available Compounds | Percent N |
| Sulfur-coated Urea | 39 |
| Urea-formaldehydes | 38 |
| Oxamide | 32 |
| Crotonylidene Diurea | 28 |
| Isobutylidene Diurea | 31 |
| Manure | |
|---|---|
| Animal Type | lb N/ton |
| Dairy | 8-12 |
| Beef | 9-14 |
| Swine | 11-17 |
| Sheep | 18-27 |
| Goat | 18-26 |
| Poultry | 19-40 |
| Horse | 10-15 |
How Nitrogen Fertilizer Affects Soil Acidity
When the nitrification process converts the ammonium ion to nitrate, hydrogen ions are released, shown by the following reaction.
| 2NH4 + | + | 3O2 |  | 2NO3 | + | 8H+ |
| Ammonium | Oxygen | Nitrifying Bacteria | Nitrate | Hydrogen |
This is a source of soil acidity (H+), so N fertilizers containing or forming ammonium-N increase soil acidity unless the plant absorbs the ammonium ion directly.
Also, nitrate is a major factor associated with leaching of such bases as calcium (Ca), magnesium (Mg), and potassium (K) from the soil. The nitrate and bases move out together. As these bases are removed and replaced by hydrogen, soils become more acid. Nitrogen fertilizers containing such strong acid-forming anions as sulfate increase acidity more than other carriers without acidifying anions.
When the mineralization process decomposes soil organic matter, the first N product is ammonium. From that point on, the same nitrification as shown above happens, creating acids.
Nitrogen carriers such as sodium nitrate and calcium nitrate leave the associated cation (Na+ or Ca+) in the soil. This makes the soil less acid.
| The table below shows how different N sources affect the acidity or basicity of soils | ||
|---|---|---|
| N Source | Percent N | Calculated Equivalent Acidity or Basicity* |
| Ammonium Sulfate | 21.0 | 535 |
| Anhydrous Ammonia | 82.0 | 180 |
| Ammonium Nitrate | 34.0 | 180 |
| Calcium Nitrate | 15.0 | 135B |
| Sodium Nitrate | 16.0 | 180B |
| Potassium Nitrate | 13.0 | 200B |
| Urea | 46.0 | 180 |
| *Pounds of calcium carbonate (CaCO3) needed to neutralize the acidity formed from 100 pounds of N. The “B” denotes basic effect. These are theoretical values and probably higher than actually takes place in the soil. | ||
Nitrogen Application Methods and Timing
Fall vs. Spring: Either method can be effective, but both have drawbacks. Fall applied N is normally more subject to losses. This can be especially significant if the N is applied to soil that is warmer than 50° F, unless nitrification inhibitors are used. This often means that fall N requires somewhat higher N rates for equal yields. While spring applied N may not have as many risks, the spring season is typically very busy and wet. This increases the risk that N may not get applied in a timely manner, or that the spring field operations may lead to excessive damage to soil structure.
Row Application or “starter fertilizer”: A small amount of row applied N, with the planter, normally increases yield potential. However, large amounts of N are not appropriate for row application for several reasons. The primary drawback of large amounts of N applied at planting, at the expense of broadcast or other methods of N application, is that not many roots contact the fertilizer band. Work by Dr. Stanley Barber, Purdue, showed that maximum yield response in corn requires that 50% of the plants roots must be in a fertilized area of soil. When less than 50% of corn roots are in the fertilized zone, yields suffer. This is confirmed by our experience with plant analysis. We receive some plant samples of crops where high rates of fertilizer are applied in narrow bands from 2” to 6” beside and somewhat below the seed. These samples are typically deficient in N as well as other nutrients.
Another version of starter fertilizer is in-furrow applications. Like 2×2 placement, this can benefit yields, but it is only suitable for very low nutrient application rates. This is because all fertilizer is salty and excess salts can damage seed germination. Therefore, most of the crops nutrient needs must be applied by other methods.
In-Season Nitrogen: This application system normally involves sidedress or foliar applications, but can also include the injection of N through an irrigation system.
Sidedress N: Sidedress N normally improves yield potential. However, there are some drawbacks. A primary concern is that adverse weather may prevent the grower from applying the sidedress N in a timely manner. If this happens, the crop may be deprived of a significant and critical portion of the seasons' N requirement.
Fertigation: Growers who use irrigation have a great opportunity to increase yields and better manage their N by injecting it into their irrigation system. The details relating to the amount and timing of this N varies according to many factors. Nitrogen applied throughout the season and according to the crop demand will normally maximize yields and minimize N losses.
Foliar Nitrogen: Foliar N can be an excellent method of adding a little “punch” to a fertilizer program. Foliar N programs are normally not strong enough, nor economical to significantly improve a weak N program. Instead, it should be thought of as a way to take advantage of opportunities to improve an already adequate program.
Soil Testing for Nitrogen
Pre-Plant, Fall: As of this writing, there is no dependable soil test for N at this time of the year. The Illinois Nitrogen test developed by Dr. Richard Mulvaney is showing some progress, but is not yet ready for general use.
Pre-Plant, Spring: As of this writing, there is no dependable soil test for N at this time of the year
Pre-Sidedress: The Pre-Sidedress Nitrogen Test (PSNT) developed Magdoff (U of VT) and made popular by Penn. State Univ. and Iowa State Univ. is an effective means of determining the amount of N required by a few selected crops. A soil sample for the PSNT must be taken to a depth of 12” in much of the Corn Belt and Eastern States, and must be taken to a depth of 24” in many areas of the dry Great Plains. There is no calibration for interpreting the PSNT taken at more shallow depths or at a different height of corn other than 12”.
| Suggested times for soil sampling for use of in-season soil nitrate tests (PSNT) on various crops | |||
|---|---|---|---|
| Crop | Scientific name | Time of soil sampling | |
| Beets | Beta vulgaris | After thinning (2- to 4-leaf sages) | |
| Broccoli | Brassica oleracea | 2 weeks after transplanting | |
| Brussels sprouts | Brassica oleracea | 2 weeks after transplanting | |
| Cabbage | Brassica oleracea | 2 weeks after transplanting | |
| Cauliflower | Brassica oleracea | 2 weeks after transplanting | |
| Celery | Apium graveolens | 2 weeks after transplanting. Sample again in about 3 to 4 weeks | |
| Corn, field | Zea mays | When plants are 15 to 25 cm (6 to 10 inches) tall | |
| Corn, sweet | Zea mays | When plants are 15 to 25 cm (6 to 10 inches) tall | |
| Cucumber | Cucumis sativus | When vines are 15 cm (6 inches) long | |
| Eggplant | Solanum melongena | At first fruit set. Sample again 3 to 4 weeks later | |
| Endive and escarole | Cichorium endivia | 2 weeks after transplanting or after thinning if direct seeded (2-4 leaf stage) | |
| Irish potato | Solanum tuberosum | When vines are 15 cm (6 inches) long. | |
| Lettuce | Lactuca sativa | 2 weeks after transplanting or after thinning if direct seeded (2-4 leaf stage) | |
| Muskmelon | Cucumis melo | When vines are 15 cm (6 inches) long | |
| Pepper | Capsicum annuum | At first fruit set. Sample again 3 to 4 weeks later | |
| Pumpkin | Cucurbita pepo | When vines are 15 cm (6 inches) long | |
| Rutabaga | Brassica napus | After thinning (2- to 4-leaf sages). | |
| Spinach | Spinacia oleracea | At 2- to 4-leaf stages. Sample again after cutting | |
| Tomato | Lycopersicon esculentum | At first fruit set. Sample again 3 to 4 weeks later | |
| Turnip | Brassica rapa | After thinning (2- to 4-leaf sages). | |
| Winter squash | Cucurbita maxima | When vines are 15 cm (6 inches) long | |
| Reference: “In-Season Soil Nitrate Testing as a guide to nitrogen Management for Annual Crops, J.R. Heckman, Rutgers Univ., Hort Technology, October-December 2002 | |||
The PSNT is a test for Nitrate-N (NO3-N). As we all know, plants also take up N in the ammonium-N (NH4-N) form, and you may ask why the NH4-N is not normally included in a PSNT test. In fact there are a few situations where the amount of NH4-N may affect the interpretation of the PSNT results. In most cases these situations are where a nitrification inhibitor has been applied or where there may be a source of NH4-N that could supply this form of N later in the summer. Some of these materials include N-Serve, DCD, Agrotain, Ammonium Thiosulfate, Sulfur-coated Urea, and other slow-release N sources.
No comments:
Post a Comment