- two solutions - 50 mL of A and 50 mL of B respectively
- a solution of 0.2M hydrochloric acid (HCl)
- a solution of 0.2M sodium hydroxide (NaOH)
- pH meter to measure pH of the solution
Experiment 1: The pH of solution A is 7.0 i.e. it’s neutral. When we add 10 mL of 0.2M HCl to it, the pH decreases to 1.5. On the other hand, when we add 10 mL of 0.2M NaOH to solution A the pH shoots up to 12.5. In both these cases we see a drastic change in pH due to either the increase or decrease of proton [Hstart superscript, plus, end superscript] concentration. Remember, pH of a solution is dependent on the concentration of hydronium ions or simply put as [Hstart superscript, plus, end superscript].
Experiment 2: Let’s see what happens when we repeat the same experiment with solution B, whose pH is also maintained at 7.0. When we add 10 mL of 0.2M HCl to it, the pH decreases by only 0.2 units to 6.8. Next, when we add 10 mL of NaOH to solution B, the pH just slightly rises to 7.2 from 7.0. In both these cases we do not see a drastic change in pH, as we observed with solution A.
So, our observation from this experiment is that solution A underwent drastic changes in pH upon addition of a strong acid or a strong base, while solution B resisted a change in pH.
Can you guess what the difference might be between solutions A and B?
Solution A is pure ‘water’, while solution B is a ‘buffer’.
How do we define a buffer?
“A buffer is an aqueous solution that resists changes in pH upon the addition of an acid or a base”. Also, adding water to a buffer or allowing water to evaporate from the buffer does not change the pH of a buffer significantly. Buffers basically constituent a pair of a weak acid and its conjugate base, or a pair of a weak base and its conjugate acid (as will be discussed next).
How do we prepare a buffer?
A buffer is essentially prepared in two ways
- mixing a large volume of a weak acid with its conjugate base (eg. acetic acid – acetate ion, CHstart subscript, 3, end subscriptCOOH – CHstart subscript, 3, end subscriptCOOstart superscript, negative, end superscript)
- mixing a large volume of weak base with its conjugate acid (eg. ammonia – ammonium ion, NHstart subscript, 3, end subscript – NHstart subscript, 4, end subscriptstart superscript, plus, end superscript)
Phosphate buffer is a very commonly used buffer in research labs. It falls under the second category. It's made up of a weak base (HPOstart subscript, 4, end subscriptstart superscript, 2, negative, end superscript) and its conjugate acid (Hstart subscript, 2, end subscriptPOstart subscript, 4, end subscriptstart superscript, negative, end superscript). The pH of a phosphate buffer is usually maintained at a physiological pH of 7.4.
How does a buffer work?
Buffer, as we have defined, is a mixture of a conjugate acid-base pair that can resist changes in pH when small volumes of strong acids or bases are added.
- When a strong base is added, the acid present in the buffer neutralizes the hydroxide ions (OHstart superscript, negative, end superscript).
- When a strong acid is added, the base present in the buffer neutralizes the hydronium ions (Hstart subscript, 3, end subscriptOstart superscript, plus, end superscript).
Let’s understand this principle through the two examples we listed above.
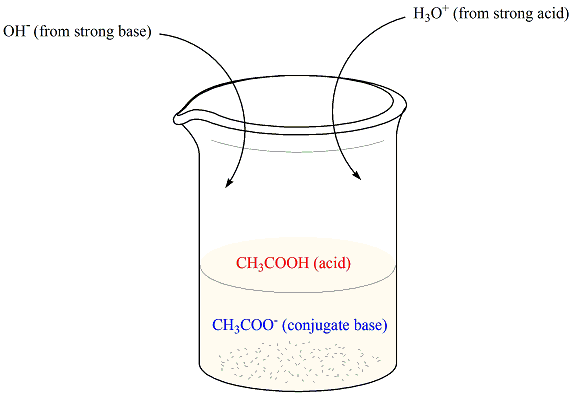
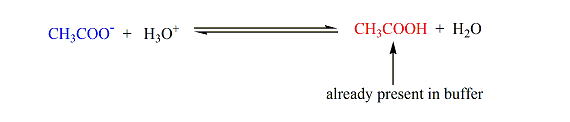
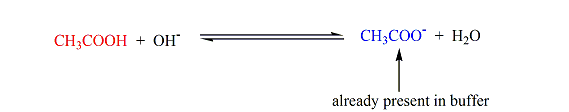
- Suppose we have a buffer that contains acetic acid (CHstart subscript, 3, end subscriptCOOH) and its conjugate base, acetate ion (CHstart subscript, 3, end subscriptCOOstart superscript, negative, end superscript), as depicted below.When a strong acid is added, the acetate ions neutralize the hydronium ions producing acetic acid (which is already a component of the buffer).On the other hand, when a strong base is added, acetic acid consumes the hydroxide ions producing acetate ions (which is also already a constituent of the buffer).
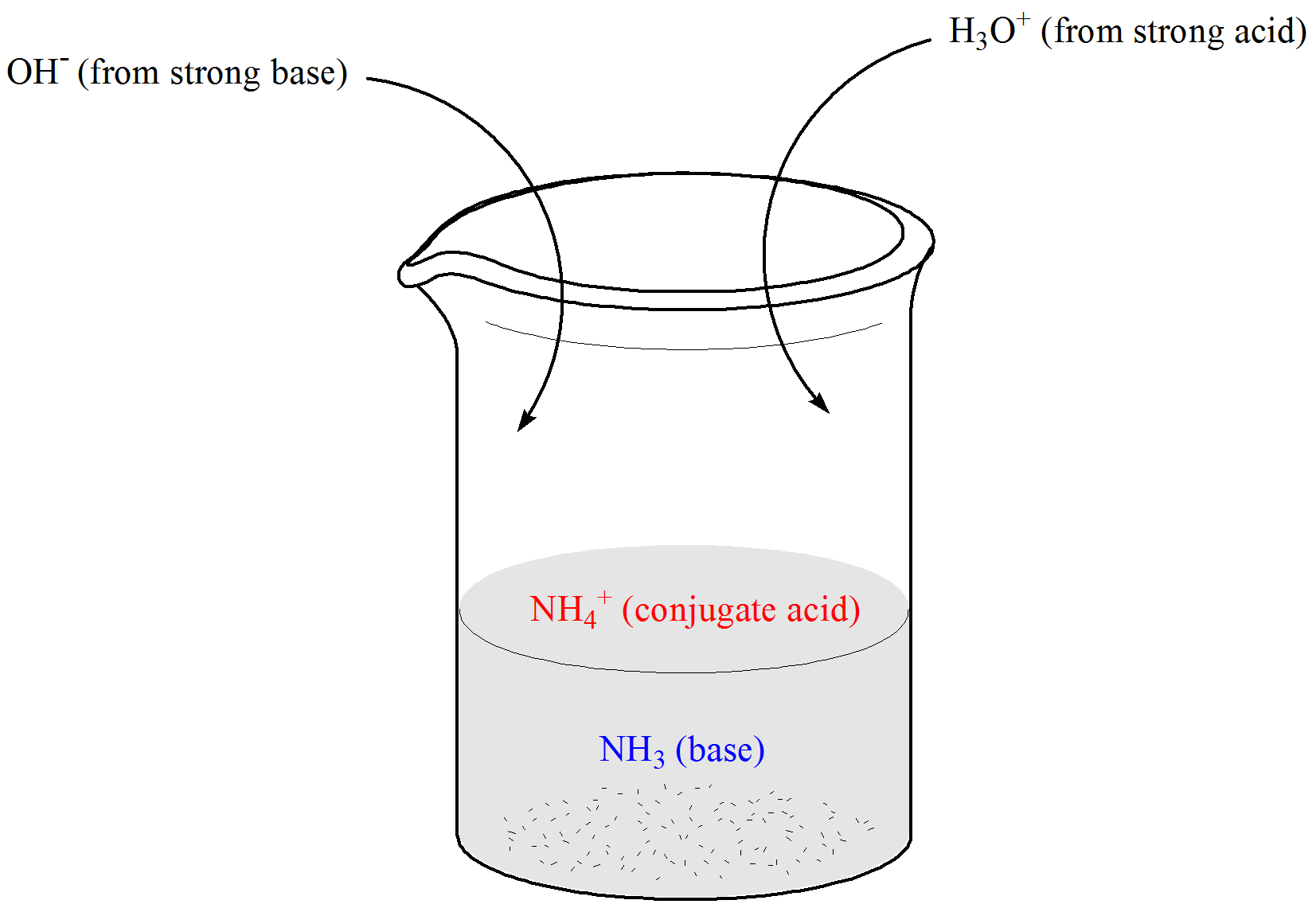
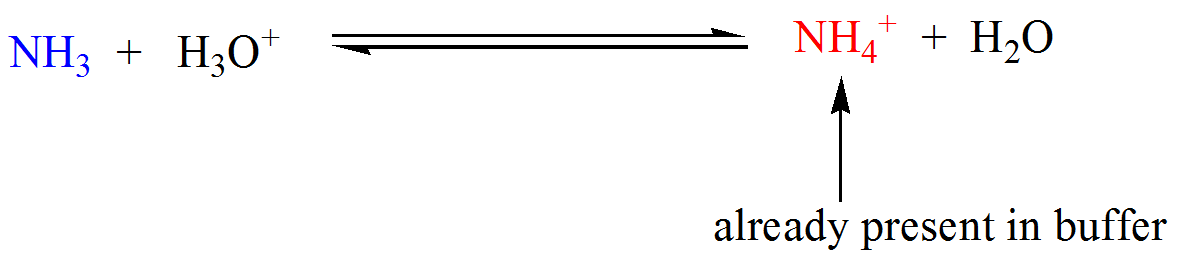
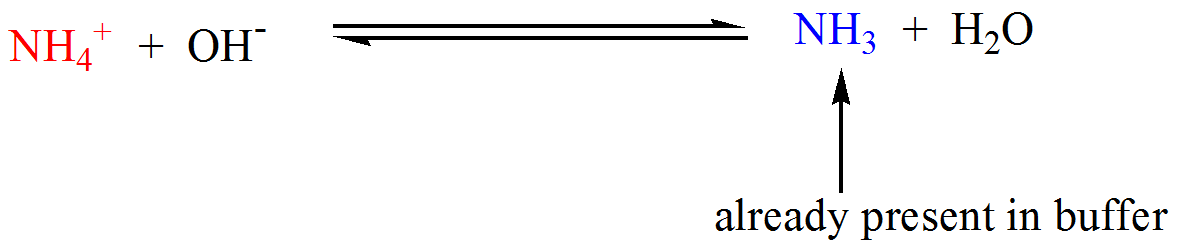
- Suppose we have a buffer that contains a weak base (NHstart subscript, 3, end subscript) and its conjugate acid, ammonium ion (NHstart subscript, 4, end subscriptstart superscript, plus, end superscript), as shown below.When a strong acid is added, ammonia neutralizes the hydronium ions producing ammonium ions (which is already a component of the buffer).On the other hand, when a strong base is added, ammonium ions consume the hydroxide ions producing ammonia (which is also already a constituent of the buffer).
So now we have an understanding of how a buffer neutralizes the hydronium ions or the hydroxide ions to resist a change in the pH upon the addition of a strong acid or a strong base respectively.
Buffer capacity of a buffer
Let’s assume our buffer is made up of a weak acid (HA) and its conjugate base (Astart superscript, negative, end superscript). In this case, the equilibrium constant of the weak acid will be represented as:
The above expression can be rearranged to give:
Since K, start subscript, a, end subscript is a constant, [Hstart subscript, 3, end subscriptOstart superscript, plus, end superscript] will depend directly on the ratio of [HA]/[Astart superscript, negative, end superscript].
The function of a buffer is to keep the pH of a solution within a narrow range. As you can notice from the above equation, the ratio of [HA]/[Astart superscript, negative, end superscript] directly influences the pH of a solution. In other words, the actual concentrations of A- and HA influence the effectiveness of a buffer.
The more Astart superscript, negative, end superscript and HA molecules available, the less of an effect the addition of a strong acid or base will have on the pH of the solution. For example, let’s see what will happen if we add a strong acid such as HCl to this buffer. Initially, the protons produced will be taken up by the conjugate base (Astart superscript, negative, end superscript)
This will slightly change the pH by altering the ratio [HA]/[Astart superscript, negative, end superscript] as [Astart superscript, negative, end superscript] and [HA] are constantly changing, but as long as there is enough Astart superscript, negative, end superscript present, the change in pH will be small. But if we keep adding HCl, eventually Astart superscript, negative, end superscript will run out. Once there is no more Astart superscript, negative, end superscript left, any additional HCl will donate its proton to water (HCl + Hstart subscript, 2, end subscriptO → Hstart subscript, 3, end subscriptOstart superscript, plus, end superscript + Clstart superscript, negative, end superscript). This will dramatically increase the concentration [Hstart subscript, 3, end subscriptOstart superscript, plus, end superscript], leading to a drastic change in the pH of the solution.
So, in order to be an effective buffer,
- The number of moles of the weak acid and its conjugate base must be significantly large compared to the number of moles of strong acid or base that may be added.
- The best buffering will occur when the ratio of [HA] to [Astart superscript, negative, end superscript] is almost 1:1. In that case pH = pKstart subscript, a, end subscript. Buffers are considered to be effective when the ratio of [HA] to [Astart superscript, negative, end superscript] ranges anywhere between 10:1 and 1:10.
Buffering system of blood
Maintaining a constant blood pH is critical for the proper functioning of our body. The buffer that maintains the pH of human blood involves a carbonic acid (Hstart subscript, 2, end subscriptCOstart subscript, 3, end subscript) - bicarbonate ion (HCOstart subscript, 3, end subscript) system.
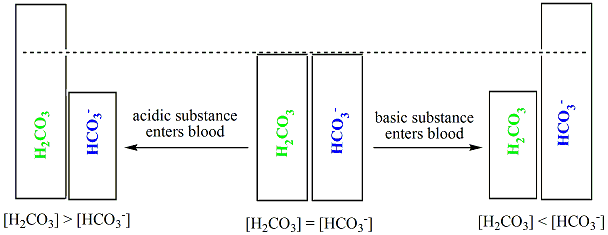
When any acidic substance enters the bloodstream, the bicarbonate ions neutralize the hydronium ions forming carbonic acid and water. Carbonic acid is already a component of the buffering system of blood. Thus hydronium ions are removed, preventing the pH of blood from becoming acidic.
On the other hand, when a basic substance enters the bloodstream, carbonic acid reacts with the hydroxide ions producing bicarbonate ions and water. Bicarbonate ions are already a component of the buffer. In this manner, the hydroxide ions are removed from blood, preventing the pH of blood from becoming basic.
As depicted below, in the process of neutralizing hydronium ions or hydroxide ions, the relative concentrations of carbonic acid (Hstart subscript, 2, end subscriptCOstart subscript, 3, end subscript) and bicarbonate ions (HCOstart subscript, 3, end subscriptstart superscript, negative, end superscript) fluctuate in the blood stream. But this slight change in the concentrations of the two components of the buffering system doesn’t have any adverse effect; the critical thing is that this buffering mechanism prevents the blood from becoming acidic or basic, which can be detrimental.
The pH of blood is maintained at ~ 7.4 by the carbonic acid – bicarbonate ion buffering system.
Why is it so critical to maintain the pH of our blood?
Believe it or not, if our blood pH goes to anything below 6.8 or above 7.8, cells of the body can stop functioning and the person can die. This is how important the optimum pH of blood is!
Enzymes are very specific in nature, and function optimally at the right temperature and the right pH; if the pH of blood goes out of range, the enzymes stop working and sometimes enzymes can even get permanently denatured, thus disabling their catalytic activity. This in turn affects a lot of biological processes in the human body, leading to various diseases.




No comments:
Post a Comment