Flow Diagram of Urea Production Process from Ammonia and Carbon-dioxide
Urea is manufactured by reacting ammonia and carbon dioxide in autoclave to form ammonium carbamate. The operating temperature is 135oC
and 35 atm pressure, the chemical reaction is endothermic reaction and
so ammonia is maintained in excess to shift the equilibrium towards urea
formation. Urea production is based on two main reactions.
1. Formation of ammonium carbamate
2. Dehydration of ammonium carbamate to produce molten urea
1. Ammonia pumping : Liquid ammonia is pumped from the multistage pump which maintain the reaction pressure in the vertical stainless steel vessel
2. Carbon dioxide compression: ammonia plant directly boost the carbon dioxide from the compression section as it readily form at the CO2 section of ammonia production plant.
3. Urea synthesis tower: It is lined with film of oxides to protect form corrosion. Catalyst bed is placed in the inner side of the autoclave structure and 180- 200 atm pressure at temperature about 180-200 deg centigrade is maintained. Plug flow operation take places and molten urea is removed from the top of the tower.
4. Distillation tower and Flash drum: This high pressure slurry is flashed to 1 atm pressure and distilled to remove excess ammonia and decomposed ammonia carbamated salts are removed and recycled.
5. Vacuum Evaporator: The solution is fed to vacuum evaporator for concentrating the slurry.
6. Prilling Tower: It is dryer where the molten slurry is passed from top of the tower into a bucket which rotates and sprinkles the slurry and air is passed from the bottom. All the moisture is removed as the urea form into granules during it journey to the bottom of the tower. This granules are sent by conveyor to the bagging section.
Biuret Formation:
Two moles of urea are converted into one mole of biuret and one mole of NH3 by heating.
2 NH2CONH2-------------> NH2CONHCONH2 + NH3
Because the biuret is injurious to germinating seeds, and pine apple and citrus trees wither when the fertilizer is sprayed on the leaf. The biuret content in fertilizer grade urea on the world market is required to be below 1.0%. biuret forms almost everywhere in urea production steps.
The following conditions are favorable for biuret formation.
• High residence times.
• High temperature.
• Low amount of water.
Process Water Treatment:
As already pointed out in the process description, the liquid effluent treatment section consists mainly of a distillation column to purify the waste water, a hydrolyser to decompose the small percentage of urea into ratio NH3and CO2 which are eventually stripped in the lower section of the same column.
The condensed vapors from first and second vacuum systems, containing urea, ammonia and CO2 are collected in the process condensate tank. In this tank the carbonate close drain is also fed by the centrifugal pump and are recycled .
A simple description which give an idea of the urea manufacturing process with plant layout:
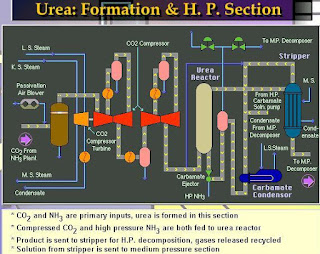
Reactor effluent:
The reactor effluent which consists of a liquid phase along with a certain percentage inerts and reactants in a vapour phase, fed to the H.P. stripper where the first carbamate decomposition occurs. The vapour phase containing most of the inert gases then flows to the carbamate condenser together with the carbamate recycle from the medium pressure section. Only before re-injecting the carbamate into the reactor, the inert gases are separated from the liquid phase-in the carbamate separator and fed to the MP decomposer.
H.P. Stripper:
It is the falling film type heat exchanger. It containing 2429 tubes with some space above the tubes and below the tubes. In the above space a 0.315m height pall rings bed arranged. A sieve tray is fitted above the packed bed. The tubes are fitted with ferrules have three tangentially drilled distribution holes. Tubes are made with titanium and shell side fluid is the medium pressure saturated steam.
The reaction product leaving the reactor flow to the steam heated falling film stripper which operates at about 144-146Kg/cm2 pressure. The liquid from the feed distributor pipe is evenly distributed onto the packed bed by means of preheated sieve having 1400 holes. The mixture is heated up as it flows into the vertical tubes of the falling film exchanger. The CO2 content of the solution is reduced by the stripping action of the ammonia as it boils out of the solution. The carbamate decomposition heat is supplied by medium pressure saturated steam, where the latent heat of condensation of saturated steam is taken by carbamate solution. In the falling film exchanger, the principle advantages are high rate of heat transfer, no internal pressure drop, short time of contact.
Decomposition is promoted by heating and stripping CO2 by vaporized excess NH3, under the same pressure level as urea reactor. Stripper used is falling film type, decomposed and vaporized gases and liquid effluent are therefore in counter-current contact and CO2 concentration in liquid is gradually reduced from the top to bottom of the stripper tube. As NH3 rich gas (CO2lean gas) rises from the lower parts of the tube, then the gas at upper parts of the tube becomes an NH3 rich gas as compared with the equilibrium composition and the decomposition reaction in liquid phase corrects the deviation from the equilibrium (the stripping effect). Decomposition at high pressure requires high temperature which means that much biuret has formed and the liquid becomes corrosive, but excess ammonia and the use of titanium in the stripper permit minimizing the problems.
The urea solution with part of inerts is coming from the bottom of the stripper enters into the medium pressure decomposition in urea purification section. The overhead gases from the top of the stripper mixed with recovered solution from medium pressure absorber and then pressurized to 180kg/cm2 in H.P. carbamate pumps and preheated in carbamate pre-heater by using steam condensate flowing to battery limits then this mixture enters tube side of carbamate condenser where heat of reaction of reaction-1 and condensation of carbamate gases is removed by production of steam at 3.5 to 4.5 kgf/cm2 on the shell side by vaporization of water. The condensate from the condenser with few inert gases is entered into the carbamate into the carbamate separator. Carbamate separator is the cylindrical empty vessel in which separation of carbamate solution from inert gases will take place, carbamate solution from bottom of separator is recycled to reactor by means of ejector.
The non-condensate gases from the top of the separator consist mainly of inert gases, with a small amount of NH3 and CO2 are passed through the split range controller to the medium pressure decomposer holder to utilize the heat of these for that decomposition.
UREA PURIFICATION:
Urea purification takes place in three stages at decreasing pressures as follows: First stage at 18kgf/cm2 Second stage at 4.5kgf/cm2 Third stage at 0.35kgf/cm2 It is pointed out that the exchangers where the urea purification occurs are called decomposer. the upper part of the medium pressure inert washing tower consists of three valve trays. Where the inert gases are subjected to a final scrubbing or washing by means of some absorption water. In this way the inerts are sent to vent stack practically free from ammonia.
Prevention of explosion hazard by gases vented to the atmosphere:
CO2 fed to the reactor normally contains a small percentage of H2,CH4 and CO in addition to inerts like N2 and Ar. These gases plus the small quantity of gases introduced into the plant with NH3 coming from B.L together with CO2 contained in passivation air could give rise to explosively problems when vented into atmosphere from MP inerts washing tower. As a matter of fact, this problem is minimized in Snamprogetti urea plants. Since the quantity of passivation air used is far lower than the one used in other processes. Thus the O2 to flammable gases ratio in the vented gases does not justify the use of a H2 removal system on the CO2 stream from B.L
CO2 fed to the reactor normally contains a small percentage of H2,CH4 and CO in addition to inerts like N2 and Ar. These gases plus the small quantity of gases introduced into the plant with NH3 coming from B.L together with CO2 contained in passivation air could give rise to explosively problems when vented into atmosphere from MP inerts washing tower. As a matter of fact, this problem is minimized in Snamprogetti urea plants. Since the quantity of passivation air used is far lower than the one used in other processes. Thus the O2 to flammable gases ratio in the vented gases does not justify the use of a H2 removal system on the CO2 stream from B.L
Purification and recovery stage at 4.5 kg/cm2:
L.P.Decomposer (LPD):
This is also the falling film type heat exchanger. It is also constructed same as to MP decomposer, the packed bed height, equipment divisions and construction are same.
The lower the pressure , the better the prevention of NH3 and CO2 loses from the system, but the recovered solution becomes weaker.Which means that excess water is recycled to the synthesis loop, the operating conditions of L.P decomposer are selected at 3.5kgf/cm2 pressure decomposer (falling film type). The gases leaving the top separator are mixed with the dilute carbon solution coining from waste water treatment and sent to the ammonia preheater, where they are practically absorbed and condensed.
The ammonia preheater is the shell and tube(1-4 pass) heat exchanger, in which LPD vapors are condensed and feed NH3 to reactor is heated. While depressurizing(drawing tube side NH3 loop, case must be taken to avoid freezing of water) solution on shell side of this preheater.
From the above condensate wit uncondensed gases then enter the LP condenser, where the residue absorption and condensation heat is removed by cooling water. The liquid phase , with remaining inert gases, is sent to the carbonate solution vessel.
The carbonate solution tank is a horizontal cylindrical vessel. It is constructed with inerts washing tower above the tank, and is located slightly taper to the ground to maintain the solution head for pumps at low level also. In shutdown followed by emptying of high pressure equipment, the recovered NH3, CO2 in low pressure stage is also stored in their tank. The level of this tank should be maintained low in order to recover all carbonate in case of shutdown.
The inert gases leaving from carbonate solution tank enters into low pressure inerts washing tower which is located on the tank with packed bed. The inerts are washed in this tower by using water in the counter current flow. The inerts which are leaving from the washing tower are vented to stack, which are practically free from NH3.
Purification and recovery stage at 0.35kg/cm2:
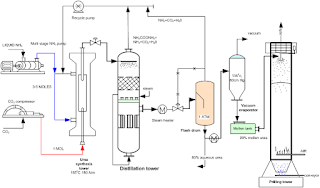
Vacuum pre-concentrator:
This is also the falling film type heat exchanger. It is also constructed same as to above decomposers with bell distributor.
The solution leaving(the bottom of low pressure decomposer is expanded at 0.35 kgf/cm2 a pressure and enters the vacuum pre-concentrator) falling film types as with the help of tangentially inlet duct. Top separator where the released flash gases are removed before the solution enters the tube bundle. Decomposition section where the last residual carbamate is decomposed and the required heat is supplied by the condensation of the gases coming from the medium pressure decomposer separator.
The gases leaving the pre-concentrator top are routed to the vacuum duct where condensation takes place. The urea solution, collected at the bottom of pre-concentrator holder is sent to the vacuum section by using centrifugal pump. The pre-concentrator is able to save a lot of pressure stream in the evaporator permits to concentrate the urea solution from 70-75% to about 85-88% wt. Low pressure section for urea production
UREA CONCENTRATION :
As it is necessary , in order to prill urea, to concentrate the urea solution up to 99.8% wt. The simplest and most widely used method is direct concentration , which consists in heating the solution under vacuum to remove water. Direct concentration is operated on the basis of the equilibrium vapour pressure of the urea solution.
Theoretically to concentrate the solution without the deposit of crystals, the operating pressure should be kept over 0.3kh/cm2 abs.., 1360C int eh second vacuum system.The urea solution coming from vacuum pre-concentrator holder is sent to the first vacuum concentrator where it is heated up to above the boiling point of that liquid at the pressure of separator. The mixed phase coming out of concentrator enters the gas-liquid separator from where vapours are extracted by the second vacuum system, while the solution fed to the prilling section by using centrifugal pump.
Both the 70-72% wt.urea solution from the L.P decomposer and the urea melt from the vacuum separator can be directed to the urea solution tank, so as to face any emergency situation in both the vacuum and prilling sections.
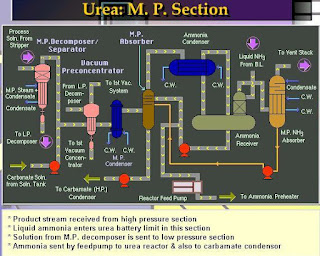
UREA PURIFICATION at M.P. Decomposer:
This is falling film type heat exchanger is divided into three parts. Top separator where the released flash gases are separated, middle decomposer where the carbamate decomposition will take place and bottom holder where the concentrated urea solution will holding. The decomposer tubes are fitted with ferrules having four tangentially distribution holes with equispaced. Packing bed of pall ring with 1.3m height and sieve plate for distribution is provided above the decomposer in separator. To promote more decomposition it is necessary to that higher temperatures or to reduce to lower levels. M.P. Decomposed is operated at 17kgf/cm2 (g) and 156-158OC decomposed heat is being supplied from outside of tube by M.P. steam and M.P. condensate.
 |
| Urea medium pressure section flow sheet |
The NH3 and CO2 rich gas leaving the top of separator are sent to vacuum pre concentrator,where they are partially absorbed in the aqueous carbonate solution coming from the urea purification section at 4.5kgf/cm2. The absorption and condensation of gases are removed by evaporating water from urea solution, thus allowing a considerable saving of L/P/ steam in the evaporation stages. Then the gases enter the M.P. condenser where the residue absorption and condensation of heat of gases is removed by cooling water. In the condenser CO2 is almost totally absorbed. The mixture from M.P. condenser flows to the medium pressure absorber.
M.P. Absorber:
It is the bubble cap tray type column contains 4 numbers of trays having bubble cap risers fitted with bell caps. It contains sparger pipe distributor at bottom. The absorber perform CO2 absorption and NH3 rectification.
Reflux NH3 is drawn as part from the NH3 booster pump and fed to the absorber on top tray and the aqueous ammonia solution which in coming from M.P.inerts washing tower is fed on the third tray by means of centrifugal pump and tray washing provision is also there.
 |
| Image of medium pressure section of urea production |
Ammonia Receiver:
It is the horizontal cylindrical vessel fitted with ammonia recovery tower. The tower is installed on the receiver with 3m packing bed height of pall rings and contain distribution sieve tray above the packed bed. The receiver is located slightly tapper to the ground.
The ammonia which is received from battery limits containing 5PPM oil. It causes the foaming in synthesis section, to avoid this foaming the oil should be separated from ammonia. In the above receiving tank, the oil will separate by density separation and comes towards the down end of the tank. This oil will drain periodically.
• The function of this receiver tank is to receive and act as a buffer storage for ammonia received from battery limit.
• To receive ammonia recovered during plant shut down.
• To receive ammonia condensed in the recovery system.
The inert gases containing residual ammonia leaving the receiver, enters the ammonia recovery tower, where the pure ammonia coming from B.L. is fed at the top of the tower. In the tower the inert gases containing NH3 and pure liquid NH3 are brought in contact with each other in a counter current flow to recover some ammonia from inerts.
The inert gases containing residual ammonia are sent to the medium pressure falling film absorber(inert washing tower) where they meet in a counter current water flow which absorbs gaseous ammonia. The heat of absorption is removed by cooling water. From the bottom of the absorber water-NH3 solution is recycled back to the medium pressure absorber by means of centrifugal pump. Tower operating at a pressure 2.5 kgf/cm2 before entering the distillation tower the process condensate is preheated in the exchangers where the heating medium is the purified condensate flowing out the tower.
Since the solution is contaminated by urea, after a first stripping in the upper part of the tower, it is pumped into the hydrolyser where the urea is decomposed by means of stream at 37 kgf/cm2 , 370oC. Before entering the hydrolyser , the solution is preheated in the exchanger with the solution coming out from the hydrolyser.
The hydrolysis reaction of urea is the opposite of that occurring in the reactor.
NH2CONH2 + H2O ------->2NH3 + CO2 + Heat
Therefore urea decomposition is favored by high temperature, low pressure and NH3 & CO2 deficiency. Also a sufficient long residence time has proved to be an important parameter. In order to eliminate NH3 and CO2 as far as possible before feeding the hydrolyser the waste water coming out from the vacuum condensers is first stripped in the column. Moreover a series of baffles in the hydrolyser provided a plug flow effect, thus avoiding back mixing. Also the continuous removal of hydrolysis reaction and this encourages the decomposition of urea.
 |
| Urea High Pressure section |
UREA PRILLING: PRILL TOWER:
It is a cylindrical vertical tower with a height of 100m, in which urea prilling takes place. It consists of prill section the top and scrapper at bottom. Prill tower contains bottom lowers(window) and top lowers(windows) also. In the prill section bucket (Tuttle type) is there. The tower is coated inside with anti corrosive plant. This is a natural draftPrill tower.
The molten urea leaving the second vacuum holder is sent to the prilling bucket by means of centrifugal pump. Bucket contains no. of holes to the wall. The urea coming out of the rotating bucket in the form of drops fall along the prilling tower and arid encounters cold air flow which causes its solidification.
The molten urea drops coming from bucket contains is at a temperature of1330C
There will be heat transfer from drops to air , thus reducing the temperature of drops and increasing the temperature of air. The heated air try to go up, due to that flow of air, some vacuum is created at the glass. The bottom air will try to cover the above vacuum thus creating the natural draft. The air will enter the prill tower through bottom lowers and vented to the atmosphere through top lowers.
The heated air with a few parts of urea dust enters the scrubbing section where the urea dust will recover from air by scrubbing of air with DM water and the free from urea dust is vented to atmosphere.
The molten urea drops from bucket falls down along the prilling tower. Due to the counter current flow of air the temperature of molten urea will decrease and form as a prill. The solid prills falling to the bottom of the prilling tower are fed to a belt conveyor by a rotary scrapper. From here they are sent to the automatic weighing machine and to the urea storage, bagging section.
DEDUSTING SYSTEM:
The urea melt coming out of the bucket in the form of droplets and while falling inside the prill tower encounters a countercurrent flow of cold air causes solidification . Hot air leaving prill tower top consists of fine urea dust and free ammonia. In order to prevent pollution caused during the process of prilling . During system has been incorporated at prill tower top. The system also recovers urea, which is recycled back into the system.
Operation:
In dedusting tank air travels in two chambers and a stainless steel partition wall which is hanging fro the top separates these two chambers. The three recirculation pumps take suction deduction chamber with the help of scrubber nozzles with an angle of 10 deg and due to this spraying action, sir is sucked into the first chamber (annual scrubbing chamber). Urea gets dissolved while exhaust air traveling from top to bottom in annular scrubber chamber and then it enters the second chamber of dedusting sump, where demister pads are provided at the top. Process condensate pump is sprayed on demister pads. By nozzles with 90deg.angle, and this system is operated by PLC (programmable logic control). Before taking DDS in line top louvers are be kept closed. Make up liquid for dedsuting sump is done by a control valve and after attaining required concentration the solution is drained to urea lumps dissolving tank. Maximum allowable urea dust to atmospheric air is 3Omg/Nm2 of air. An energy-efficient process for urea synthesis must fulfill the following parameters.
• High conversion efficiency of CO2 in urea synthesis reactor, in order to minimize the heat required for decomposition of unconverted carbamate.(Achieved by optimization of parameters in the urea reactor).
• Efficient decomposition of carbamate and efficient separation of carbamate decomposition products(CO2 and NH3 ), as well as of excess ammonia .(Optimization of process parameters in the stripper and decomposer)
• Maximum recovery and efficient utilization of heat formed by absorption and reaction of NH3 and CO2 released from the stripper and decomposition. (Optimization of process parameters in the carbamate condenser ,the MP decomposes and MP absorber).
No comments:
Post a Comment