Chemical indicator, any substance that gives a
visible sign, usually by a colour change, of the presence or absence of a
threshold concentration of a chemical species, such as an acid or an
alkali in a solution. An example is the substance called methyl yellow,
which imparts a yellow colour to an alkaline solution.
INDICATORS 18.5




For acid-base titrations, organic compounds that exhibit different colors in solutions of different acidities; used to determine the point at which reaction between two solutes is complete.
Example of chemical indicators to the left illustrates the color of the solution dependent on PH.
From this indicator example, you can see that the qualitative action of an acid-base indicator is as follows:
Since indicators are usually large organic molecules with complicated formulas the chemical formula is often abbreviated as Hln.
Looking back on the chart, it is evident that different chemical indicators disassociate at different pHs. The following video gives examples of the color methyl orange, methyl red, bromothymol blue, litmus, phenolphthalein and indigo carmine display in acidic hydrochloric acid and basic sodium hydroxide.
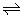 H+ + In- lies exactly in the middle.
H+ + In- lies exactly in the middle.
If you use the information from the chart in previous section "how indicators work" you can derive an equilibrium constant as follows:
When the equilibrium lies exactly in the center [ln-] = [Hln] in which case you can cancel them out of the equation giving Ka=H+.
From Ka=H+ you can log both sides leading to pKa=pH
The consequence of this answer is that the indicator will change color when the pH is the same value as its pKa value. Hence, indicators have different regions of operation. As the change in pH is usually large at the equivalence point this means that provided the pH change takes place through the pKa of the indicator then it can be used for a titration.



Table of Contents
Definition of Chemical Indicators
How Do Indicators work? (18.5.1)
pKa of Indicators (18.5.2)
Phenolphthalein (18.5.3)
Naturally Occurring pH Indicators
Universal Indicator
Definition of Chemical Indicators

For acid-base titrations, organic compounds that exhibit different colors in solutions of different acidities; used to determine the point at which reaction between two solutes is complete.
Example of chemical indicators to the left illustrates the color of the solution dependent on PH.
How Do Indicators work? (18.5.1)
Indicators work because they are weak acids which, when in solution, exist in equilibrium with their conjugate base. The acid and its conjugate base each have different colors, and as the equilibrium shifts from one direction to the other, the color of the indicator solution changes. As an example, the equilibrium of bromothymol blue can be represented by the equation:| Hln (aq) |
<==> |
H+ (aq) |
+ |
ln- (aq) |
|
| yellow |
|
blue |
|
From this indicator example, you can see that the qualitative action of an acid-base indicator is as follows:
| Hln (aq) |
<==> |
H+ (aq) |
+ |
ln- (aq) |
|
| coulor A |
|
colour b |
|
Since indicators are usually large organic molecules with complicated formulas the chemical formula is often abbreviated as Hln.
Looking back on the chart, it is evident that different chemical indicators disassociate at different pHs. The following video gives examples of the color methyl orange, methyl red, bromothymol blue, litmus, phenolphthalein and indigo carmine display in acidic hydrochloric acid and basic sodium hydroxide.
pKa of Indicators (18.5.2)
As indicators have a different color when in the molecular form to when in the ionic form it seems logical to assume that the point of changing colour will be when there is 50% of both forms present i.e. the equilibrium HIn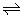 H+ + In- lies exactly in the middle.
H+ + In- lies exactly in the middle.If you use the information from the chart in previous section "how indicators work" you can derive an equilibrium constant as follows:
Ka = |
[H+][In-] |
| [HIn] |
When the equilibrium lies exactly in the center [ln-] = [Hln] in which case you can cancel them out of the equation giving Ka=H+.
From Ka=H+ you can log both sides leading to pKa=pH
The consequence of this answer is that the indicator will change color when the pH is the same value as its pKa value. Hence, indicators have different regions of operation. As the change in pH is usually large at the equivalence point this means that provided the pH change takes place through the pKa of the indicator then it can be used for a titration.
Phenolphthalein (18.5.3)
Phenolphthalein is commonly used in titration experiments dealing with weak acids and strong bases because of its noticeable clear to pink color change when the solution goes past a pH of 8.2. The phenolphthalein molecule is colorless however the phenolphthalein ion is pink. When a base is added to the phenolphthalein, the molecule ions equilibrium shifts to the right, leading to more ionization as H+ ions are removed. This is predicted by Le Chatelier's principle. |
| Phenolphthalein in an alkaline solution. Note the fuchsia color. |
 |
| Phenolphthalein in an acidic solution. Note the clearness. |
Naturally Occurring pH Indicators
Some plant parts contain chemicals from the naturally-colored anthrocyanin family of compounds. They are red in acidic solutions and blue in basic. Anthocyanins can be extracted from leaves (red cabbage); flowers (geranium, poppy, or rose petals); berries (blueberries, blackcurrant); and stems (rhubarb). Litmus, the indicator commonly found on strips, is derived from species of lichens. |
| Parmelia sulcata, one of the species of lichens that litmus is derived from. |
No comments:
Post a Comment