Gas Chromatography VS Liquid Chromatography
What is gas chromatography and liquid chromatography?
Gas chromatography is a chromatographic method that uses gas as a mobile phase. Sample flows through the gas system and is gasified before finally entering a chromatographic column filled with a filler to achieve effective separation. Gas chromatography has the advantages of high sensitivity, small sample usage, strong separation ability, good selectivity, wide application, and fast analysis.
Liquid chromatography is a liquid phase with a packed bed, paper, and thin plates as stationary phases. It is operated at room temperature and does not need to consider the influence of sample volatility and thermal stability during substance separation. Therefore, liquid chromatography can be used to separate and analyze high thermal sensitivity, difficult vaporization and non-volatile substances. According to its separation mechanism, liquid chromatography can be divided into four types: adsorption chromatography, partition chromatography, ion exchange chromatography, and gel chromatography. Its working principle is similar to the classic liquid chromatography. The main difference is in the size of the packed particles. It is mainly used to separate large molecular weights, high boiling point and organic compounds of different polarities. High pressure is required to transport the mobile phase, so it is also called high pressure liquid chromatography.
How to read gas chromatography and liquid chromatography?
Chromatograms obtained from either gas or liquid chromatography can be interpreted the same way. Data output by the detector is displayed as a line graph, and the number of detected compounds changes with time. The peaks of the most volatile compounds appear first on the graph. Subsequent peaks on the graph represent a gradual decrease in volatility of the original mixture. Researchers can use these chromatograms to further break down the chemical properties of the sample mixture. The ratio of the peak size is related to the amount of substance in the sample. Area under a mountain peak is used to determine the sample size. For example, to determine a component in a sample, a standard sample of known concentration needs to be analyzed first. Compare the retention time and peak area on the chromatogram of the standard to the testing sample. Target compound in the sample is then obtained.
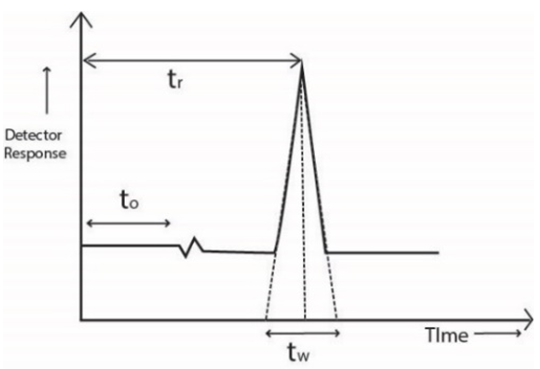
Figure 1. Retention Time (tr)
How does gas chromatography and liquid chromatography work?
After entering the vaporization chamber in gas chromatography, sample solution will enter the chromatographic column, transferred by the carrier gas (the carrier gas is usually nitrogen or helium). Different components are separated in the chromatographic column and finally flow out of the column. The activities in the column is detected by a detector. As each component is detected successively, chromatographic signal is recorded by a recorder, an integrator or a data processing system.
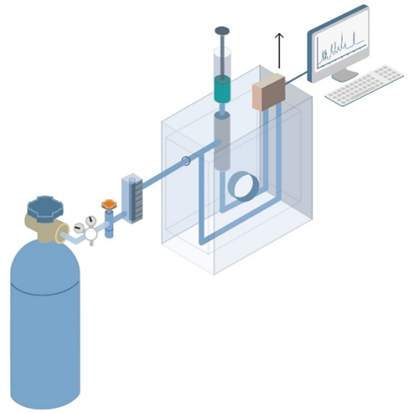
Figure 2. The process of gas chromatography
For liquid chromatography, the liquid mobile phase flows through an infusion pump, mixes with the sample solution, and finally flows out of the column. Adsorption and separation are performed in the column. The detector will eventually convert all components into electrical signals, or the corresponding sample peaks, at the chromatographic testing station.
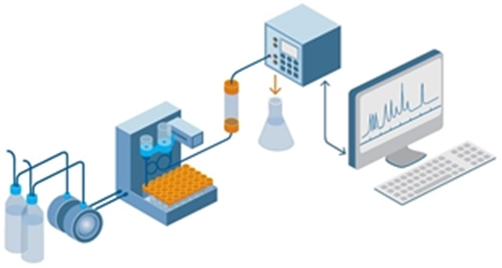
Figure 3. The process of liquid chromatography
The applications of gas chromatography and liquid chromatography
Gas chromatography can be used for chemical separation experiments of chiral compounds, research on the separation and determination of parabens in paraben food preservatives, separation of different pesticides, detecting doping in plasma,and research on the detection of chemical agents of environmental pollutants, etc. Liquid chromatography is mainly used in food detection applications, such as the detection of toxic and harmful substances, microbial products, nutrients and additives in food, research on potential biomarkers of pesticide pollution in the environment, and determination of toxins in plasma and urine, etc.
References
1. Adissu Alemayehu Asfaw, Kris Wolfs, Ann Van Schepdael, et al. Development and validation of a thermal desorber gas chromatography method for determination of residual solvents in drug loaded albumin. Journal of Pharmaceutical and Biomedical Analysis, 2020, 179.
2. Tiantian Shi, Meiling Qi, Xuebin Huang. High-resolution performance of triptycene functionalized with polycaprolactones for gas chromatography[J]. Journal of Chromatography A, 2020, 1614.
3. Gabriella Ambrosio, Jan Felix Joseph, Bernhard Wuest, et al. Detection and quantitation of ecdysterone in human serum by liquid chromatography coupled to tandem mass spectrometry. Steroids, 2020, 157.
4. Zahra Gharari, Khadijeh Bagheri, Hossein Danafar, et al. Simultaneous determination of baicalein, chrysin and wogonin in four Iranian Scutellaria species by high performance liquid chromatography. Journal of Applied Research on Medicinal and Aromatic Plants, 2020, 16.
No comments:
Post a Comment