Dissolved Oxygen
What is dissolved oxygen?
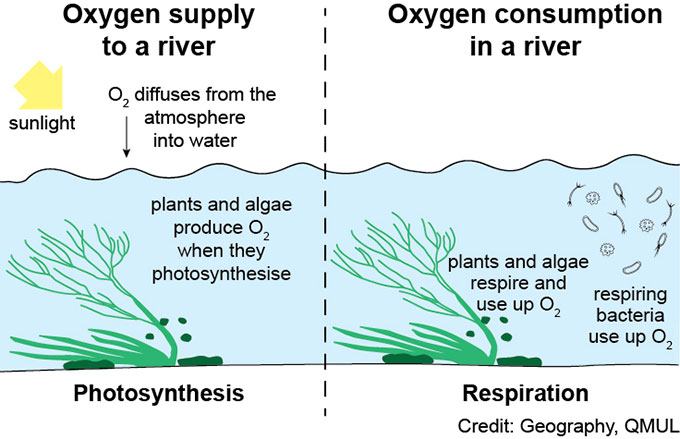
Dissolved oxygen is a measure of the amount of gaseous oxygen (as O2) dissolved in water. Oxygen enters the river water from the atmosphere (via diffusion), from groundwater , and also as a by-product of the photosynthesis of aquatic plants. Oxygen levels in water can be expressed in units of concentration, as mg/L, or in relation to the maximum amount of oxygen that can be dissolved in the water at a certain temperature, called ‘% saturation’.
Why do we measure dissolved oxygen?
Fish and zooplankton need oxygen in water in order to breathe. Good oxygen levels are critical for the health of a river system. Slow flowing, polluted river water is often associated with low oxygen conditions, which cannot support much life. As we will see dissolved oxygen is closely linked to organic pollution and nutrient enrichment, so can tell us quite a bit about the health of a river.
What are the natural controls on dissolved oxygen?
Oxygen levels in river water are a balance between oxygen supply (from the atmosphere and by photosynthesis) and oxygen consumption due to the respiration of plants, animals and microbes. Bacteria use organic matter (including plant and animal remains) in water as a food source. The more organic matter, the greater the number of bacteria respiring in the water, and hence the more oxygen that gets used up by the bacteria. Therefore, excess organic matter in rivers can cause low oxygen conditions.
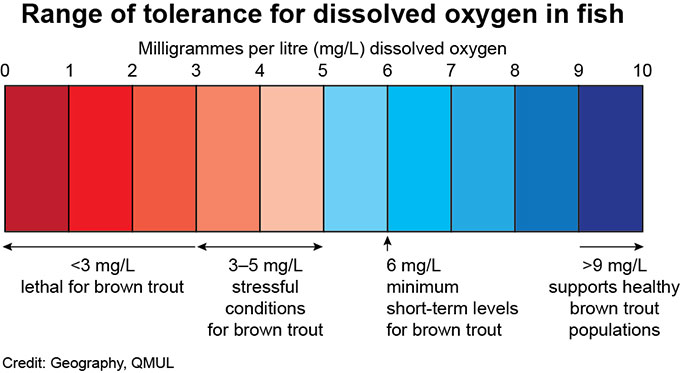
The solubility of oxygen in water decreases as water temperature increases. This means that oxygen levels can show seasonal variations, with less oxygen in summer when water temperature are high, and more oxygen in winter when temperatures are low. This can have important consequences for fish health.
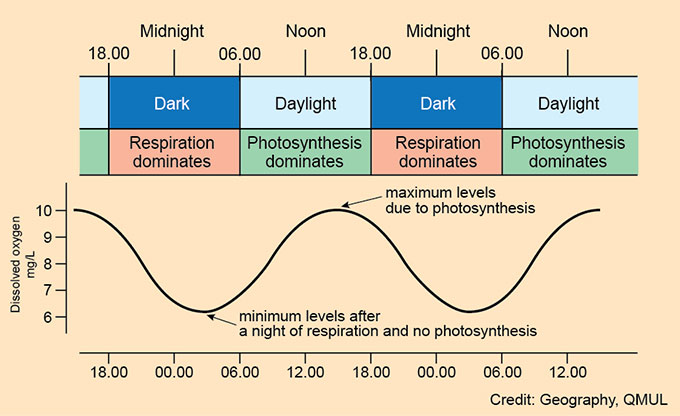
Chalk streams are valued for their aquatic plants which grow over the spring and summer months. These plants will photosynthesise by day, and respire at night causing marked daily changes in the dissolved oxygen content of the river. The rate of photosynthesis will change depending on the strength and duration of sunlight, so a sunny day will be associated with higher rates of photosynthesis compared to a cloudy one.
How can human activity change dissolved oxygen?
Sewage (also called domestic wastewater) contains human faeces which is made up of organic matter. One of the purposes of sewage treatment is to break down the organic matter under controlled conditions so that the organic matter is not released into the river where it can cause harm to people and river life. Any release of raw sewage into a river is accompanied by a decrease in dissolved oxygen content because bacteria, which use the sewage as a food source, grow in number and respire. This respiration rapidly uses up oxygen in the water and can lead to fish kills.
In the UK we have combined sewer systems which collect both stormwater from roads, roofs of buildings and car parks when it rains, as well as sewage from our homes and businesses. When rainfall is heavy, hard surfaces in the catchment rapidly transport water to our sewer systems. Overflows from sewers into rivers can occur if there is not enough capacity in the sewage treatment works to handle these stormwater flows.
Misconnections in the underground pipe network can also mean that sewer pipes are incorrectly linked to overflows to rivers. These misconnections can be quite common in built up areas, and result in water high in organic matter reaching our rivers which can cause problems with oxygen levels.
No comments:
Post a Comment