
IPCS INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 19
Hydrogen Sulfide
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Published under the joint sponsorship of the United Nations
Environment Programme, the International Labour Organisation, and the
World Health Organization
World Health Organization
Geneva, 1981
ISBN 92 4 154079 6
(c) World Health Organization 1981
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. For rights of reproduction or
translation of WHO publications, in part or in toto, application
should be made to the Office of Publications, World Health
Organization, Geneva, Switzerland. The World Health Organization
welcomes such applications.
The designations employed and the presentation of the material in
this publication do not imply the expression of any opinion whatsoever
on the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city or area or
of its authorities, or concerning the delimination of its frontiers or
boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR HYDROGEN SULFIDE
1. SUMMARY AND RECOMMENDATIONS FOR FURTHER STUDIES
1.1. Summary
1.1.1. Properties and analytical methods
1.1.2. Sources of hydrogen sulfide
1.1.3. Environmental levels and exposures
1.1.4. Effects on experimental animals
1.1.5. Effects on man
1.1.5.1 General toxicological considerations
1.1.5.2 Occupational exposure
1.1.5.3 Exposure of the general population
1.1.6. Evaluation of health risks
1.2. Recommendations for further studies
2. PROPERTIES AND ANALYTICAL METHODS
2.1. Chemical and physical properties
2.2. Atmospheric chemistry
2.3. Sampling and analytical methods
2.3.1. The methylene blue method
2.3.2. Gas chromatography with flame photometric detection
2.3.3. Automatic monitors in stationary field settings
2.3.4. Direct reading portable detection systems
2.3.5. Manual collection and analysis of air samples in
occupational settings
3. SOURCES OF HYDROGEN SULFIDE
3.1. Natural sources
3.2. Sources associated with human activity
4. ENVIRONMENTAL LEVELS AND EXPOSURE
4.1. Concentrations in outdoor air
4.2. Concentrations in work places
5. EFFECTS ON EXPERIMENTAL ANIMALS
6. EFFECTS ON MAN
6.1. General toxicological considerations
6.2. Occupational exposure
6.3. General population exposure
7. EVALUATION OF HEALTH RISKS FROM EXPOSURE TO HYDROGEN SULFIDE
7.1. Exposure levels
7.2. Experimental animal studies
7.3. Effects of occupational exposure
7.4. Effects of general population exposure
7.5. Guidelines for the protection of public health
REFERENCES
ANNEX
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR HYDROGEN SULFIDE
Members
Dr M. Argirova, Institute of Hygiene & Occupational Health, Sofia,
Bulgaria
Dr G. C. N. Jayasuriya, National Science Council, Colombo, Sri Lanka
(Vice-Chairman)
Dr H. Kappus, Department of Pharmacology, Medical Institute for
Environmental Hygiene, Düsseldorf, Federal Republic of Germany
Professor M. Katz, York University, Faculty of Sciences, Department of
Chemistry, Downsview, Ontario, Canada
Mr. K. Rolfe, Department of Health, Environmental Laboratory,
Auckland, New Zealand (Rapporteur)
Dr H. Savolainen, Institute of Occupational Health, Helsinki, Finland
Professor R. P. Smith, Department of Pharmacology and Toxicology,
Dartmouth Medical School, Hannover, NH, USA (Chairman)
Dr S. Tarkowski, Institute of Occupational Medicine, Industrial
Toxicology Branch, Lodz, Poland
Mr E. Tolivia, Department of Health and Welfare, Mexico City, Mexico
Dr V. V. Vashkova, Sysin Institute of General and Communal Hygiene,
Moscow, USSR
Temporary Advisers
Dr T. H. Milby, Environmental Health Associates, Inc., Berkeley, CA,
USA
Dr R. C. Spear, Department of Biomedical and Environmental Health
Sciences, School of Public Health, University of California,
Berkeley, CA, USA
Representatives of other Organizations
Dr D. Djordjevic, International Labour Office, Geneva, Switzerland
Dr S. Salem Milad, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme, Geneva,
Switzerland
Mrs M. Th. van der Venne, Commission of the European Communities,
Directorate General of Employment and Social Affairs, Health and
Safety Directorate, Luxembourg
Secretariat
Dr A. David, Office of Occupational Health, Division of
Noncommunicable Diseases, World Health Organization, Geneva,
Switzerland
Dr Y. Hasegawa, Environmental Health Criteria and Standards, Division
of Environmental Health, World Health Organization, Geneva,
Switzerland (Secretary)
Dr H. W. de Koning, Environmental Health Technology and Support,
Division of Environmental Health, World Health Organization,
Geneva, Switzerland
Mr G. Ozolins, Environmental Health Criteria and Standards, Division
of Environmental Health, World Health Organization, Geneva,
Switzerland
NOTE TO READERS OF THE CRITERIA DOCUMENTS
While every effort has been made to present information in the
criteria documents as accurately as possible without unduly delaying
their publication, mistakes might have occurred and are likely to
occur in the future. In the interest of all users of the environmental
health criteria documents, readers are kindly requested to communicate
any errors found to the Division of Environmental Health, World Health
Organization, Geneva, Switzerland, in order that they may be included
in corrigenda which will appear in subsequent volumes.
In addition, experts in any particular field dealing with in the
criteria documents are kindly requested to make available to the WHO
Secretariat any important published information that may have
inadvertently been omitted and which may change the evaluation of
health risks from exposure to the environmental agent under
examination, so that the information may be considered in the event of
updating and re-evaluation of the conclusions contained in the
criteria documents.
ENVIRONMENTAL HEALTH CRITERIA FOR HYDROGEN SULFIDE
A WHO Task Group on Environmental Health Criteria for Hydrogen
Sulfide met in Geneva from 24 to 28 March 1980. Mr G. Ozolins,
Associate Manager, Environmental Health Criteria and Standards, opened
the meeting on behalf of the Director-General. The Task Group reviewed
and revised the second draft of the criteria document and made an
evaluation of the health risks from exposure to hydrogen sulfide.
The first and second drafts were prepared jointly by Dr T. H.
Milby of the Environmental Health Associates, Inc., Berkeley, CA, USA,
and Dr R. C. Spear of the Department of Biomedical and Environmental
Health Sciences, University of California, Berkeley, CA, USA. The
comments on which the second draft was based were received from the
national focal points for the WHO Environmental Health Criteria
Programme in Australia, Belgium, Czechoslovakia, Finland, Federal
Republic of Germany, Mexico, New Zealand, Poland, USA and USSR, and
from the International Labour Organisation, Geneva, the International
Centre for Industry and Environment, France, and the International
Petroleum Industry Environmental Conservation Association, London.
Comments were also received from Professor M. Katz (Canada) and
Professor R. Lilis (USA). Some comments were received after the second
draft had been prepared and were reviewed by the Task Group during its
meeting. These comments were from the national focal points for the
WHO Environmental Health Criteria Programme in Japan and the United
Kingdom and from the Commission of the European Communities,
Luxembourg, and the International Union of Pure and Applied Chemistry,
London.
The collaboration of these national institutions, international
organizations and individual experts is gratefully acknowledged.
Without their assistance this document could not have been completed.
This document is based primarily on original publications listed
in the reference section. However, several recent publications broadly
reviewing health aspects of hydrogen sulfide have also been used,
including those of the National Research Council, USA (1979) and NIOSH
(1977).
Details of the WHO Environmental Health Criteria Programme,
including some of the terms frequently used in the documents, can be
found in the introduction to the publication "Environmental Health
Criteria 1 - Mercury", published by the World Health Organization,
Geneva, 1976, now also available as a reprint.
The following conversion factors have been used in this document:
hydrogen sulfide: 1 ppm = 1.5 mg/m3, 1 mg/m3 = 0.7 ppm.
* *
*
Financial support for the publication of this criteria document
was kindly provided by the Department of Health and Human Services
through a contract from the National Institute of Environmental Health
Sciences, Research Triangle Park, North Carolina, USA - a WHO
Collaborating Centre for Environmental Health Sciences.
1. SUMMARY AND RECOMMENDATIONS FOR FURTHER STUDIES
1.1 Summary
1.1.1 Properties and analytical methods
Hydrogen sulfide is a colourless gas with a characteristic odour
that is soluble in various liquids including water, alcohol, ether,
and solutions of amines, alkali carbonates, and bicarbonates. It can
undergo a number of oxidation reactions to yield principal products
consisting of sulfur dioxide, sulfuric acid, or elemental sulfur.
Reaction rates and oxidation products depend on the nature of the
oxidizing agent.
The methylene blue colorimetric method has acceptable specificity,
accuracy, and sensitivity for hydrogen sulfide determinations, and is
generally recognized as a standard analytical procedure. It has been
used successfully, in automatic continuous monitoring, but
sophisticated maintenance facilities and highly trained technicians
are required for this method. Gas chromatography coupled with flame
photometric detection is an alternative method for hydrogen sulfide
determination, either as a laboratory method or for continuous
monitoring in stationary field settings.
Most of the direct-reading methods of hydrogen sulfide
determination in the occupational environment are susceptible to
various forms of interference. However, methods employing chemical
detector tubes appear to be useful in occupational settings, where
hazardous levels of hydrogen sulfide can occur. Under these
conditions, reliability and accuracy compensate for a certain lack of
specificity.
1.1.2 Sources of hydrogen sulfide
Hydrogen sulfide is one of the principal compounds involved in the
natural cycle of sulfur in the environment. It occurs in volcanic
gases and is produced by bacterial action during the decay of both
plant and animal protein. It can also be produced by bacteria through
the direct reduction of sulfate. Significant concentrations of
hydrogen sulfide occur in some natural gas fields and in geothermally
active areas.
Hydrogen sulfide can be formed whenever elemental sulfur or
certain sulfur-containing compounds come into contact with organic
materials at high temperatures. In industry, it is usually produced as
an undesirable by-product, though it is an important reagent or
intermediate in some processes. Hydrogen sulfide occurs as a
by-product in: the production of coke from sulfur-containing coal, the
refining of sulfur-containing crude oils, the production of carbon
disulfide, the manufacture of viscose rayon, and in the Kraft process
for producing wood pulp.
1.1.3 Environmental levels and exposures
Though concentrations of hydrogen sulfide in urban areas may
occasionally be as high as 0.050 mg/m3 (0.033 ppm) with averaging
times of 30 min-1 h, they are generally (below 0.0015 mg/m3
(0.001 ppm). Peak concentrations as high as 0.20 mg/m3 (0.13 ppm)
have been reported in the neighbourhood of point sources. In a
geothermal area, 1-h mean concentrations of up to 2 mg/m3 (1.4 ppm)
have been observed. When hydrogen sulfide was accidentally released in
an incident in Poza Rica, Mexico, in 1950, the number of deaths that
followed indicated that exposure levels probably exceeded
1500-3000 mg/m3 (1000-2000 ppm).
It is believed that workers are not usually exposed to hydrogen
sulfide concentrations above the occupational exposure limits of
10-15 mg/m3 (7-10 ppm) (8-h time-weighted average) adopted by many
governments. There are, however, numerous reports of accidental
exposures to concentrations that have ranged from 150 mg/m3
(100 ppm) to as high as 18 000 mg/m3 (12 000 ppm). Such massive
exposures to hydrogen sulfide have resulted either from leaks in
industrial gas streams containing high levels of hydrogen sulfide or
from the slow, insidious accumulation of hydrogen sulfide in low-lying
areas. The second case may arise when hydrogen sulfide of biogenic
origin is generated from such sources as sewage disposal plants and
cesspools.
1.1.4 Effects on experimental animals
In experimental animals, the effects of high doses of hydrogen
sulfide and high doses of cyanide are very similar. Cyanide inhibits
the enzyme cytochrome c oxidase [EC 1.9.3.1] a, thereby
interfering with tissue use of oxygen to the point where metabolic
demands cannot be met. Hydrogen sulfide also exhibited an inhibitory
action on a purified preparation of cytochrome c oxidase.
Results of studies on a number of animal species including canary,
rat, guineapig, cat, dog, and goat showed that inhalation of hydrogen
sulfide at a concentration of 150-225 mg/m3 (100-150 ppm)
resulted in signs of local irritation of eyes and throat after many
hours of exposure; at 300-450 mg/m3 (200-300 ppm), eye and mucous
membrane irritation appeared after 1 h inhalation and slight general
effects after prolonged inhalation; at 750-1050 mg/m3 (500-700 ppm),
local irritation and slight systemic signs appeared within 1 h and
a The numbers within brackets following the names of enzymes are
those assigned by the Enzyme Commission of the Joint IUPAC-IUB
Commission on Biochemical Nomenclature.
death after several hours; at 1350 mg/m3 (900 ppm), serious systemic
effects appeared in less than 30 min and death within 1 h; at
2250 mg/m3 (1500 ppm), collapse and death occurred within 15-30 min;
and, at 2700 mg/m8 (1800 ppm), there was immediate collapse with
respiratory paralysis, and death. There is little information on the
effects on experimental animals of long-term, low-level exposure to
hydrogen sulfide gas.
1.1.5 Effects on man
1.1.5.1 General toxicological considerations
Hydrogen sulfide is both an irritant and an asphyxiant gas. Its
direct irritant action on the moist tissues of the eye produces
keratoconjunctivitis, known as "gas eye". When inhaled, hydrogen
sulfide exerts an irritant action throughout the entire respiratory
tract, although the deeper structures suffer the greatest damage. A
consequence may be pulmonary oedema. At concentrations of
1500-3000 mg/m3 (1000-2000 ppm), hydrogen sulfide gas is rapidly
absorbed through the lung into the blood, which initially induces
hyperpnoea (rapid breathing). This is followed by respiratory
inactivity (apnoea). At higher concentrations, hydrogen sulfide exerts
an immediate paralysing effect on the respiratory centres. Death due
to asphyxia is the certain outcome, unless spontaneous respiration is
re-established or artificial respiration is promptly provided. This
sequence of events represents the most important toxic effect of
hydrogen sulfide.
Acute hydrogen sulfide intoxication can be defined as the effects
from a single exposure to massive concentrations of hydrogen sulfide
that rapidly produce signs of respiratory distress. Concentrations
exceeding about 1500 mg/m3 (1000 ppm) produce such acute effects.
Subacute hydrogen sulfide intoxication is the term applied to the
effects of continuous exposure for up to several hours to
concentrations ranging from 150 to 1500 mg/m3 (100-1000 ppm). In
this range of exposure, eye irritation is the most commonly observed
effect. However, some reports have indicated that the threshold for
eye irritation occurs after several hours of exposure to hydrogen
sulfide at levels of 16-32 mg/m3 (10.5-21.0 ppm). Pulmonary oedema
may be a more important and potentially fatal complication of subacute
hydrogen sulfide intoxication. Chronic intoxication is a largely
subjective state characterized by fatigue and believed by some to be a
consequence of intermittent exposure to hydrogen sulfide
concentrations of 75-150 mg/m3 (50-100 ppm). Not all research
workers accept the existence of such a condition.
The characteristic "rotten egg" odour of hydrogen sulfide is well
known. The threshold of perception of this odour varies considerably
depending on individual sensitivity, but, under laboratory conditions,
it ranges from 0.0008 to 0.20 mg/m3 (0.0005-0.13 ppm). Above about
225 mg/m3 (150 ppm), the gas exerts a paralysing effect on the
olfactory apparatus, thus neutralizing the value of its odour as a
warning signal. At these concentrations, the odour of the gas has been
reported to be sickeningly sweet.
1.1.5.2 Occupational exposure
Exposure to hydrogen sulfide in high concentrations occurs in
numerous occupations. Workers in the oil, gas, and petrochemical
industries are occasionally exposed to hydrogen sulfide in
concentrations sufficient to cause acute intoxication. In one survey
of the petrochemical industry, among 221 cases of hydrogen sulfide
poisoning, the overall mortality was 6% and a high proportion of
victims exhibited neurological signs and symptoms. Forty percent of
all cases required some form of respiratory assistance; 15% developed
pulmonary oedema.
Persistent sequelae following acute intoxication have been
reported among workers in a number of occupations including sewer
workers, chemical plant employees, farmers, shale-oil workers, and
laboratory attendants. Most victims who develop sequelae experience a
state of unconsciousness during the acute phase of their illness.
However, sequelae following acute intoxication without unconsciousness
have also been reported.
1.1.5.3 Exposure of the general population
Several episodes of general population exposure to hydrogen
sulfide have been reported. The effects of such exposure have ranged
from minor nuisance to serious illness and death. In a small community
adjacent to an oilfield installation, large quantities of hydrogen
sulfide were released into the air when an oilfield flare
malfunctioned. Three hundred and twenty persons were hospitalized, 22
of whom died. Nine exhibited manifestations of pulmonary oedema. Four
victims developed neurological sequelae. The air levels of hydrogen
sulfide were not measured.
A community of 40 000 people, located in the vicinity of a large
geothermal field, was exposed in some areas to hydrogen sulfide levels
in air (measured on a continuous basis over a 5-month period)
exceeding 0.08 mg/m3 (0.05 ppm), for, on average, 35% of the time.
Although fatal cases of hydrogen sulfide intoxication associated with
improper ventilation in geothermal steam-heated dwellings in this area
were occasionally reported until 1962, the major problem has been the
nuisance caused by the odour of the gas. In a moderately sized
community, hydrogen sulfide was released from a small industrial waste
lagoon resulting in a 1-h average concentration of hydrogen sulfide in
air of 0.45 mg/m3 (0.3 ppm). Complaints were mostly related to the
odour of hydrogen sulfide gas. However, the severity of complaints of
nausea, vomiting, headache, loss of appetite, and disturbed sleep
exceeded the mere nuisance level.
No community studies of the long-term, low-level effects of
hydrogen sulfide exposure have been reported.
1.1.6 Evaluation of health risks
Hydrogen sulfide in ambient air in concentrations of the order of
the odour threshold has not been shown to have any significant
biological activity in man or animals. In controlled laboratory
studies, the odour threshold for hydrogen sulfide has been reported to
range from 0.0008 to 0.20 mg/m3 (0.0005-0.13 ppm). Little
information is available on the odour detection limits for hydrogen
sulfide either under experimental field conditions or in the ambient
air. However, the Task Group considered that a level of 0.008 mg/m3
(0.005 ppm) averaged over 30 min should not produce odour nuisance in
most situations. In the occupational setting, the earliest toxic
response appears to be eye irritation, which has been reported to
occur at 16-32 mg/m3 (10.5-21.0 ppm) after several hours' exposure.
The occupational exposure guidelines for hydrogen sulfide recommended
by the Task Group included the adoption of a level of 10 mg/m3
(7 ppm) as a workshift time-weighted average value together with a
short-term exposure limit of 15 mg/m3 (10 ppm), the latter to be
determined as a 10-min or less, average value.
1.2 Recommendations for Further Studies
Measurements of hydrogen sulfide concentrations in the ambient air
should be included in studies of the levels in air of other common
gaseous contaminants, such as the oxides of sulfur and nitrogen.
Studies in areas remote from man-made emission sources would provide
background data for the development of models for long-distance
transport and diffusion, for the evaluation of biological decay
processes from natural sources, and for developing a clearer
understanding of global sulfur cycles. More studies are required to
elucidate processes involving chemical and photochemical oxidation
reactions of hydrogen sulfide. Studies are also necessary to develop
methods for the personal dosimetry measurement of hydrogen sulfide
that do not require wet chemical techniques.
Studies should be conducted in experimental animals on the
cumulative neural effects of repeated and/or continuous long-term
hydrogen sulfide exposure at concentrations that induce subacute or
chronic intoxication. The cardiac sequelae after acute intoxication
should be investigated in intact animals and in those with pre-induced
cardiac damage. Studies of the toxicokinetics of absorbed hydrogen
sulfide are needed and other studies should be initiated to test for
the metabolic generation of hydrogen sulfide from sulfur-containing
organic compounds.
Case studies should be made of patients who have suffered acute
hydrogen sulfide intoxication to examine the long-term effects on the
myocardium. Efforts should be made to estimate the dose of hydrogen
sulfide associated with acute poisoning. Prospective studies of new
workers to investigate the effects of long-term exposure to
concentrations of hydrogen sulfide likely to be encountered in the
work place would be valuable. These studies should include
considerations of morbidity and mortality, the incidence of cancer and
teratogenic effects, and studies of changes in pulmonary function with
time. Continuing environmental studies should play a major part in
these prospective studies, in order to provide dose-response data,
where possible. Similar studies should be initiated among the general
population in a geothermal area, taking advantage of the natural
conditions provided, for example, by the situation in Rotorua, New
Zealand.
2. PROPERTIES AND ANALYTICAL METHODS
2.1 Chemical and Physical Properties
Hydrogen sulfide is a flammable colourless gas with the
characteristic odour of rotten eggs. It burns in air with a pale blue
flame and, when mixed with air, its explosive limits are 4.3% to 46%
by volume. Its autoignition temperature is 260°C. The relative
molecular mass of hydrogen sulfide is 34.08. Its density is
1.5392 g/litre at 0°C and 760 min. The ratio density of hydrogen
sulfide compared with air is 1.19. One gram of hydrogen sulfide
dissolves in 187 ml of water at 10°C, in 242 ml of water at 20°C, in
314 ml of water at 30°C, and in 405 ml of water at 40°C (calculated
from Weast, 1977-78). It is also soluble in alcohol, ether, glycerol,
and in solutions of amines, alkali carbonates, bicarbonates and
hydrosulfides. The vapour pressure of hydrogen sulfide is
18.75 × 105 Pa at 20°C and 23.9 × 105 Pa at 30°C. Its melting
point is -85.5°C and its boiling point is -60.3°C (Macaluso, 1969;
Windholz, 1976).
Hydrogen sulfide can undergo a large number of oxidation
reactions, the type and rate of the reaction and the oxidation
products depending on the nature and concentration of the oxidizing
agent. The principal products of such reactions are sulfur dioxide,
sulfuric acid, or elemental sulfur. Aqueous solutions of chlorine,
bromine, and iodine may react with hydrogen sulfide to form elemental
sulfur. In the presence of oxides of nitrogen, the oxidation of
hydrogen sulfide in the gas phase may result in the formation of
sulfur dioxide or sulfuric acid but, in aqueous solution (pH 5-9), the
primary product is elemental sulfur (Macaluso, 1969).
Hydrogen sulfide dissociates in aqueous solution to form 2
dissociation states involving the hydrosulfide anion (HS-) and the
sulfide anion (S=). The pKa in 0.01-0.1 mol/litre solutions at 18°C
is 7.04 for HS- and 11.96 for S=. At the physiological pH of 7.4,
about one-third of the total sulfide remains as the undissociated acid
and about two-thirds as the HS- ion. The undissociated hydrogen
sulfide in solution is in dynamic equilibrium at the air-water
interface with gaseous hydrogen sulfide (National Research Council,
USA, 1977).
2.2 Atmospheric Chemistry
The atmospheric chemistry of hydrogen sulfide and other sulfur
compounds involves chemical and photochemical oxidation reactions of
emissions from both natural and man-made sources. The eventual
oxidation products are sulfuric acid (H2SO4) and/or sulfate ion
(SO4=).
There have been relatively few studies of the persistence and
conversion of hydrogen sulfide under atmospheric conditions.
Krasovitskaja and her co-workers (Krasovitskaja et al., 1965) studied
the relationship between concentrations of hydrogen sulfide, sulfur
dioxide, carbon monoxide, and hydrocarbons, and the distance from
their industrial sources. Hydrogen sulfide concentrations dropped by a
factor of 2 between the immediate neighbourhood of the source and a
2.5 km radius. A further decrease in concentration ranging from 30% up
to a factor of 8 occurred between the 2.5 km and 20 km radii. These
decreases were, in general, greater than those observed for any of the
other pollutants measured. Andersson et al. (1974) reported studies
concerning the photolysis of hydrogen sulfide and its reaction with
sulfur dioxide, as well as its reactions with atomic and molecular
oxygen and with ozone.
Junge (1963) calculated that the residence time of hydrogen
sulfide was approximately 1.7 days in the presence of an ozone level
of 0.05 mg/m3. A similar residence time was estimated by Katz (1977)
using data from the global budget of the sulfur cycle presented by
Kellogg et al. (1972). Robinson & Robbins (1970) found a residence
time in relatively clean air of about 2 days, compared with only about
2 h in a polluted urban atmosphere.
Considerably lower values than those of the previously mentioned
investigators, based on the global sulfur budget, have been presented
by Granat et al. (1976), on the basis of a very much lower release of
sulfur compounds from the biological decay of organic matter from land
and sea. Clearly, this represents a subject that requires further
studies involving the measurement of atmospheric concentrations in
relatively clean areas on land and elsewhere.
2.3 Sampling and Analytical Methods
Because levels of hydrogen sulfide in air, which are of interest
as far as human health in concerned, range from highly concentrated
industrial gas streams to ambient air pollution levels, numerous
analytical methods have been applied. A recent review of current
methods can be found in Becker (1979). A further complication in
determining air levels of hydrogen sulfide is that according to the
air monitoring application, sampling may be on either an intermittent
or a continuous basis. Intermittent samples have been taken in plastic
bags, evacuated bottles, Tutweiler burets (Shaw, 1940), and detector
tubes (West, 1970). Continuous samples have been taken by exposing
chemically treated paper tapes (Sanderson et al., 1966; Peregud et
al., 1971) or ceramic tiles (Gilardi & Manganelli, 1963) to air, by
pumping air through a lead acetate solution, by bubbling air through
impingers containing absorbing or colorimetric solutions (Goldman et
al., 1940), and by using long-duration detector tubes or electronic
detectors (West, 1970; ACGIH, 1972; Thompkins & Becker, 1976).
Qualitative hydrogen sulfide detection has been based on the
blackening of coins, keys, lead-based paint, and lead-acetate treated
papers. More recently, direct reading instruments have been developed
that make real-time monitoring possible. In some of these instruments,
a 2-step absorption-reaction procedure is involved whereas in others,
the gas reacts directly with, for example, metal-oxide-coated chips,
the electrical properties of which change in response to various gas
concentrations. Gas chromatographic methods of analysis have been
developed and are particularly used by oil and gas production
companies (Stevens et al., 1971). A recent report of the US National
Institute for Occupational Safety and Health (NIOSH, 1977) summarizes
the current situation with special reference to automatic and/or
portable samplers. The report concludes that wet chemical methods are
attractive because of their specificity and precision, but that they
are less desirable on the basis of the portability and maintenance
characteristics of the equipment. Direct reading solid state devices,
on the other hand, are portable and relatively rugged but are often
nonspecific and susceptible to cross-sensitivities.
However, the practical importance of such cross-sensitivities
depends a great deal on the type of study. In ambient air pollution
studies in which hydrogen sulfide can be excepted to be in the
0.0015-0.075 mg/m3 (0.001-0.050 ppm) concentration range,
interference may be of much greater practical concern than in
industrial settings in which concentrations may reach from 30 to
75 mg/m3 (20 to 50 ppm) or more, on occasion, and in which the
presence and identity of interfering compounds are often known.
Because of the diversity of circumstances under which hydrogen
sulfide has to be determined, only the two principal methods of
analysis for hydrogen sulfide are described in detail in the following
section. Questions of sampling and analysis suitable for several
specific practical applications are also discussed.
2.3.1 The methylene blue method
The methylene blue colorimetric method has been evaluated and
recommended by various research workers (Jacobs, 1965; Bamesberger &
Adams, 1969) and by some institutions such as the US National
Institute for Occupational Safety and Health (NIOSH, 1977). This
method has also been proposed by the International Organization for
Standardization (ISO, 1978). The Intersociety Committee of the
American Public Health Association has published detailed procedures
of this method for assessing hydrogen sulfide both in the ambient air
and in workplace air (Intersociety Committee, 1977a).
Although light, mercaptans, sulfides, nitrogen dioxide, and sulfur
dioxide can cause interference, and instruments incorporating both the
absorption and reaction functions are not portable, the methylene blue
method appears to combine adequate specificity with good accuracy and
precision and extreme sensitivity. It can be used with either manual
or automatic sample collectors and, in the latter case, with
continuous sampling, levels of hydrogen sulfide as low as 0.003 g/m3
(0.002 ppm) can be detected (Levaggi et al., 1972).
In the Intersociety Committee method, hydrogen sulfide is
collected by aspirating a measured volume of air through an alkaline
suspension of cadmium hydroxide. The sulfide is precipitated as
cadmium sulfide to prevent air oxidation of the sulfide, which occurs
rapidly in an aqueous alkaline solution. Arabinogalactan is added to
the cadmium hydroxide slurry to minimize the photodecomposition of the
precipitated cadmium sulfide. The collected sulfide is subsequently
determined by spectrophotometric measurement of the methylene blue
produced by the reaction of the sulfide with a strongly acid solution
of N, N-dimethyl- p-phenylenediamine and ferric chloride. The
analysis should be completed within 24-26 h of collection of the
sample.
This method is intended for the determination of hydrogen sulfide
concentrations in the range of 0.0012-0.1 mg/m3 (0.0008-0.07 ppm).
For concentrations above 0.08 mg/m3 (0.05 ppm), the sampling period
can be reduced or the volume of liquid increased either before or
after aspirating. Excellent results have been obtained using this
method for air samples containing hydrogen sulfide concentrations in
the range of 7.5-75 mg/m3 (5-50 ppm). This method is also useful for
the measurement of source emissions. For example, 100 ml cadmium
sulfide-arabinogalactan medium in Greenberg-Smith impingers and 5-min
sampling periods have been used successfully.
The methylene blue reaction is highly specific for sulfide at the
low concentrations usually encountered in ambient air. Strong reducing
agents (e.g., sulfur dioxide) inhibit colour development. Even
solutions containing several micrograms of sulfide per millilitre show
this effect and must be diluted to eliminate colour inhibition. If
sulfur dioxide is absorbed to give a sulfite concentration in excess
of 10 µg/ml, colour formation is retarded. Up to 40 µg/ml of this
interference, however, can be overcome by adding 2-6 drops
(0.5 ml/drop) of ferric chloride instead of a single drop for colour
development, and extending the reaction time to 50 min. On the other
hand, nitrogen dioxide gives a pale yellow colour with the sulfide
reagents at concentrations of 0.5 µg/ml or more. No interference is
encountered when 0.57 mg/m3 (0.3 ppm) of nitrogen dioxide is
aspirated through a midget impinger containing a slurry of cadmium
hydroxide-cadmium sulfide-arabinogalactan. If hydrogen sulfide and
nitrogen dioxide are simultaneously aspirated through cadmium
hydroxide-arabinogalactan, slurry, lower results are obtained,
probably because of the gas-phase oxidation of the hydrogen sulfide
prior to precipitation as cadmium sulfide.
Using permeation tubes as a source of hydrogen sulfide, a relative
standard deviation of 3.5% and a recovery of 80% have been
established. The overall sampling and analytical precision is 12.1%
relative standard deviation.
Hydrogen sulfide is readily volatilized from an aqueous solution,
when the pH is below 7.0. Alkaline aqueous sulfide solutions are very
unstable because the sulfide ion is rapidly oxidized by exposure to
the air.
Cadmium sulfide is not appreciably oxidized, even when aspirated
with pure oxygen in the dark. However, exposure of an impinger
containing cadmium sulfide to laboratory or more intense light sources
produces immediate and variable photodecomposition. Losses of 50%-90%
of sulfide have been routinely reported by a number of laboratories.
Even though the addition of arabinogalactan to the absorbing solution
controls the photodecomposition, it is necessary to protect the
impinger from light at all times. This is achieved by the use of low
actinic glass impingers, paint on the exterior of the impingers, or
aluminium foil wrapping.
The Intersociety Committee (1977a) has described the apparatus,
reagents, and calibration methods suitable for use with midget
impinger samplers and appropriate for determining air concentrations
of 0.0012-0.1 mg/m3 (0.0008-0.07 ppm). In this range, calibration is
recommended using PTFE permeation tubes. In the higher concentration
range relevant to workroom air, the National Institute for
Occupational Safely and Health recommends that calibration be carried
out using commercially available cylinders of hydrogen sulfide in dry
nitrogen (NIOSH, 1977).
2.3.2 Gas chromatography with flame photometric detection
An Intersociety Committee method also exists for the determination
of hydrogen sulfide using gas chromatography (Intersociety Committee,
1977b). This method requires the use of a gas chromatograph equipped
with a flame photometric detector. A narrow-band optical filter
selects the 394 ± 5 mm sulfur line. Gas chromatography separates
sulfur compounds of low relative molecular mass before detection, and
thereby allows individual quantitative measurement of sulfur-
containing gases such as hydrogen sulfide, sulfur dioxide, methyl
mercaptan, and dimethyl sulfide.
In situations where minimal quantities of reduced sulfur compounds
other than hydrogen sulfide are present, flame photometry can be used
directly, in which case the hydrogen sulfide concentration is
approximately the same as the total sulfur compounds measured. An
absorbant is usually required to selectively remove sulfur dioxide,
when flame photometry is used without separation of individual
compounds by gas chromatography before detection.
The limits of detection of this method, at twice the noise level
for hydrogen sulfide, sulfur dioxide, methyl mercaptan, and dimethyl
sulfide, range from 0.005 to 0.013 mg/m3 (0.0035-0.009 ppm).
Sensitivity can be increased by the use of sample concentration
techniques such as a freeze-out loop in the gas chromatograph sampling
line. The upper limit of detection is 0.5 mg/m3 (0.35 ppm) but it
can be extended by sample dilution to higher ranges, if necessary.
Although no data are available on the precision and accuracy of the
method for atmospheric samples, repetitive sampling of standard
reference gases containing a hydrogen sulfide concentration of
0.08 mg/m3 (0.055 ppm) and a sulfur dioxide concentration of
0.104 mg/m3 (0.036 ppm) gave a relative standard deviation of less
than 3% of the amount present (Intersociety Committee, 1977b).
An advantage of flame photometric detection is that chemical
solutions are not necessary, and the only required reagent is hydrogen
for the flame. However, the need for a compressed hydrogen supply may
be a disadvantage in certain situations. The analyser is calibrated
using hydrogen sulfide, sulfur dioxide, methyl mercaptan, and dimethyl
sulfide permeation tubes, and a dual-flow gas dilution device capable
of producing reference standard atmospheres as low as the limits of
detection of the method. Because the photomultiplier tube output is
logarithmically proportional to the sulfur concentration, conversion
can be done by either plotting the response against concentration on a
logarithmic scale or by using a logarithmic-linear amplifier. Using
either of these techniques, the range has been established at
approximately 0.13-0.5 mg/m3 (0.09-0.35 ppm) with a 1% noise level
(Intersociety Committee, 1977b).
Several commercial flame photometric detection analysers are now
available (with and without separation of the sulfur compounds by gas
chromatography before detection). This method of analysis for hydrogen
sulfide is suitable for use as a laboratory method for calibration
purposes or for continuous monitoring in stationary field settings.
2.3.3 Automatic monitors in stationary field settings
Paper tapes impregnated with lead acetate have been widely used
for making measurements in the field (Denmead, 1962; Thom & Douglas,
1976; Institute of Hygiene and Epidemiology, 1978). A measured volume
of air is filtered through the tape and the optical density of the
discoloured area is compared with an unexposed area of the same tape.
Numerous criticisms of these procedures have been reported and it is
clear that the presence of any substance capable of oxidizing the lead
sulfide can lead to substantial errors (Sanderson et al., 1966).
Various modifications of the basic method have been suggested to
minimize errors and increase sensitivity by Siu et al. (1971),
including the substitution of mercuric chloride for lead acetate as
proposed by Paré (1966). Natusch et al. (1974) evaluated various paper
tape methods and concluded that tapes impregnated with silver nitrate
are highly suitable for the determination of hydrogen sulfide
concentrations in the range of 0.0015-75 mg/m3 (0.001-50 ppm).
Moreover, they state that monitors using silver nitrate tape are
simple, specific, portable, capable of unattended operation and
inexpensive. However, silver nitrate tape systems remain subject to
photodecomposition, an important deficiency for many field uses.
Continuous monitors based on various wet chemical procedures have
been developed. For example, some sulfur dioxide monitors based on
amperometric principles can be used to monitor hydrogen sulfide by
replacing a silver screen (which normally filters out H2S) with a
barium acetate scrubber that removes any sulfur dioxide in the
influent airstream. These devices are reported to have good
maintenance characteristics and are suitable for use in remote areas.
However, like most continuous instruments based on wet chemistry, they
are relatively expensive (Lawrence Berkeley Laboratory, 1976).
Continuous monitors using the methylene blue method have also been
developed (Levaggi et al., 1972). These units are attractive in that
they possess the inherent sensitivity of the methylene blue method,
but they require sophisticated support facilities and highly trained
personnel for reliable operation. In this respect, the newer metal
oxide-coated-chip semiconductor devices appear promising for field
use. However, there are few published reports of field experience to
date (Thompkins & Becker, 1976).
2.3.4 Direct-reading portable detection systems
Semiquantitative methods of hydrogen sulfide detection based on
lead acetate-treated papers on tiles have been reported and are said
to be sensitive to levels of about 1 mg/m3 (0.7 ppm) (Gilardi &
Manganelli, 1963). However, long-duration detector tubes for hydrogen
sulfide, suitable for use in the occupational environment, are now
available, which are inexpensive and responsive over a wide range of
concentrations (1-84 mg/m3; 0.7-56 ppm) and overcome some of the
deficiencies of the lead acetate detectors (Leichnitz, 1977). The US
National Institute for Occupational Safety and Health has investigated
the quality and reproducibility of detector tubes available from
various US manufacturers and reports that it is possible to obtain
tubes that meet the quality specifications of the Institute (Johnson,
1972). Detector tubes for hydrogen sulfide are susceptible to
interference from other sulfides, sulfur dioxide, and nitrogen
dioxide, but, generally such interference would result in false
positive readings.
Various portable direct-reading hydrogen sulfide meters available
on the US market have been evaluated by the National Institute for
Occupational Safety and Health (Thompkins & Becker, 1976). These
instruments are intended principally for industrial hygiene surveys
and, in particular, for ascertaining the degree of general compliance
with occupational health standards for hydrogen sulfide. The
instruments surveyed operated on solid-state electrochemical
principles, wet alectrochemical principles, and in one case, on a
photoionization principle. In terms of response time, calibration
stability, and reliability, the photoionization instrument was
regarded as superior, but it was the least specific of the instruments
evaluated. The solid-state instruments tended to have slow response
times and accuracy deficiencies but were very reliable and rugged. The
wet alectrochemical meters ranked highly in terms of accuracy,
response time, and calibration stability but were somewhat less
reliable.
2.3.5 Manual collection and analysis of air samples in occupational
settings
In 1943, the American Public Health Association Sub-Committee on
Chemical Methods in Air Analysis recommended collecting hydrogen
sulfide with cadmium chloride in 2 simple petticoat bubblers in series
followed by titration with iodine, using starch as an indicator or
using an excess of iodine and back-titrating with sodium thiosulfate
solution (Goldman et al., 1943). In 1965, the AIHA Analytical Guide
(AIHA, 1965) listed 3 methods for determining hydrogen sulfide in air:
(a) iodine oxidation using a Tutweiler buret; (b) cadmium sulfate and
iodine in a midget impinger; and (c) formation of cadmium sulfide
colloid using 2 midget impingers in series followed by conversion to
methylene blue. The iodine methods are susceptible to interference at
hydrogen sulfide levels expected in occupational settings. More
recently, to ascertain employee exposure to hydrogen sulfide, the
National Institute for Occupational Safety and Health recommended the
collection of breathing-zone samples with a midget impinger and
analysis by the methylene blue method (NIOSH, 1977).
In recent years, there have been developments in the use of solid
adsorbents for the collection of sulfur gases. This technique for
sample collection could be used in association with the gas
chromatography using flame photometric detection for the measurement
of hydrogen sulfide and other low relative molecular mass or self-
containing gases (Black et al., 1978). This could ultimately lead to
the development of solid-state personal dosimeters for hydrogen
sulfide as an alternative to those that involve wet chemistry.
3. SOURCES OF HYDROGEN SULFIDE
3.1 Natural Sources
Hydrogen sulfide is one of the principal compounds involved in the
natural sulfur cycle in the environment (National Research Council,
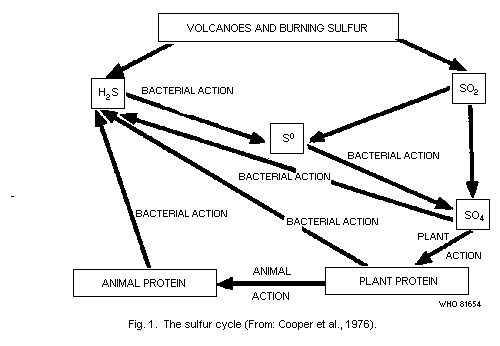
USA, 1979). As indicated in Fig. 1, it occurs in volcanic gases and is
produced by bacterial action during the decay of both plant and animal
protein (Cooper et al., 1976). Many bacteria, fungi, and actinomycetes
release hydrogen sulfide into the environment during the decay of
compounds containing sulfur-bearing amino acids and in the direct
reduction of sulfate. The heterotroph Proteus vulgaris is an example
of a common bacterium that produces hydrogen sulfide, when grown in
the presence of protein (National Research Council, USA, 1979).
The reduction of sulfate to hydrogen sulfide can be accomplished
by members of 2 genera of anaerobic bacteria, Desulfovibrio and
Desulfotomaculum. The organic substrates for these organisms are
usually short chain organic acids that are provided by the
fermentative activities of other anaerobic bacteria or more complex
organic material. Hence, hydrogen sulfide production can be expected
in conditions where oxygen is depleted, organic material is present,
and sulfate is available (National Research Council, USA, 1979).
From a microbiological point of view, the production of hydrogen
sulfide is balanced by processes involving a variety of bacteria,
found in soil and water, that can oxidize hydrogen sulfide to
elemental sulfur. Among these are the filamentous bacteria, Beggiatoa
and Thiothrix. Photosynthetic bacteria belonging to the families
Chromatiaceae and Chlorobiaceae oxidize hydrogen sulfide to elemental
sulfur and sulfate in the presence of light and the absence of oxygen.
Reduced sulfur compounds are also oxidized in nature by members of the
genus Thiobacillus. The end result of this oxidative activity is the
production of sulfate which, once formed, is extremely stable to
further chemical activity in nature (National Research Council, USA,
1979).
As a result of these various biogeochemical processes, hydrogen
sulfide occurs in and around sulfur springs and lakes and is almost
continuously present as an air contaminant in some geothermally active
areas.
3.2 Sources Associated with Human Activity
There are various circumstances under which naturally occurring
hydrogen sulfide is released by human activity. For example, hydrogen
sulfide occurring in association with natural gas and/or crude oil
deposits in some areas may be released during extraction and drilling
operations. The sulfur content of crude oils ranges from 0 to 5% and
some natural gas deposits have been reported to comprise up to 42%
 hydrogen sulfide (Espach, 1950). Coals can contain sulfur levels of up
to 80 g/kg and, occasionally, conditions arise in which hydrogen
sulfide is formed within such deposits. Thus, special precautions must
be taken in some mining operations as well as in the drilling and
extraction of natural gas and crude oils with significant sulfur
content.
Hydrogen sulfide can also be released by activities surrounding
the development and use of geothermal resources. At the Cerro Prieto
geothermal power generating plant in Baja California, Mexico, for
example, hydrogen sulfide levels are sufficiently high to necessitate
special ventilation to protect electrical systems, and alarms for the
protection of personnel (Mercado, 1975).
During industrial operations, hydrogen sulfide can be formed
whenever elemental sulfur or certain sulfur-containing compounds come
into contact with organic materials at high temperatures. It is
usually produced as an undesirable by-product, though it is also used
as an important reagent or desirable intermediate compound in some
industrial processes such as the manufacture of sulfides, sodium
hydrosulfide, and various organic sulfur compounds. Examples of
processes in which hydrogen sulfide occurs as a by-product include the
production of coke from sulfur-containing coal, the production of
carbon disulfide, the manufacture of viscose rayon in the Kraft
process for producing wood pulp (Macaluso, 1969) and sulfur extraction
by the Frasch process.
In refining sulfur-containing crude oils, about 80%-90% of the
divalent sulfur compounds of hydrogen and carbon are converted to
hydrogen sulfide. Both the hydrogen sulfide produced and that
occurring in other industrial, geothermal, or natural gas streams can
be recovered by one of a number of processes that can be classified as
either absorption-desorption processes or processes involving
oxidation to oxides or to elemental sulfur. The bulk of hydrogen
sulfide recovered in industrial processes is used to produce elemental
sulfur or sulfuric acid (Macaluso, 1969).
Large quantities of hydrogen sulfide are used in the production of
heavy water, which is employed as a moderator in some nuclear power
reactors. The process is based on enrichment of the deuterium content
of water by hydrogen sulfide in a gas/liquid ion exchange system,
followed by separation of heavy water and water by fractional
distillation (McGraw-Hill Encyclopedia of Science and Technology,
1960).
In the tanning industry, hydrogen sulfide is produced in the
process by which hair or wool is removed from the hides. This
typically involves deliming by adding ammonium chloride or ammonium
sulfate followed by pickling with sulfuric acid, and takes place in
large rotating drums. The gases evolved, including hydrogen sulfide,
are released from the drums on opening the hatches either to add
chemicals or to unload the treated hides, and also from the waste
waters (ILO, 1971).
As in the natural environment, hydrogen sulfide can be generated
by bacterial action in industrial or community settings in malodorous
and sometimes dangerous amounts.
In some countries, such as India and Sri Lanka, hydrogen sulfide
is produced in the process by which coconut fibres are separated from
the husk. This procedure involves the decomposition of the husks in
shallow ponds. The hydrogen sulfide is produced as a result of
microbiological decay processes.
4. ENVIRONMENTAL LEVELS AND EXPOSURE
4.1 Concentrations in Outdoor Air
There are few published data on either natural background or urban
air levels of hydrogen sulfide. Robinson & Robbins (1970) estimated
the average ambient air level of hydrogen sulfide to be 0.0003 mg/m3
(0.0002 ppm). This estimate supports the data of Minster (1963) who,
when sampling over a 2´-year period in northwest London, reported that
air levels of hydrogen sulfide were generally below 0.00015 mg/m3
(0.0001 ppm), under clear, fresh conditions. Minster (1963) also
reported that average summer levels ranged from 0.00015 to 0.0007
mg/m3 (0.0001-0.0005 ppm) and average winter levels from 0.0007 to
0.0015 mg/m3 (0.0005-0.001 ppm). Data collected from this same
station during the London fog of December 1962 indicated a hydrogen
sulfide concentration of up to 0.046 mg/m3 (0.033 ppm) on 6 December
during heavy fog (each sampling time was 32 min).
Measurements summarized by the US National Air Pollution Control
Administration show concentrations ranging from below 0.001 mg/m3 to
0.006 mg/m3 (0.0007 to 0.0042 ppm) at various urban locations in the
USA in the period 1951-64 (Miner, 1969). However, as sensitive and
standardized methods of sampling and analysis for hydrogen sulfide
were lacking during this period, there is some doubt about the
reliability of these data. Furthermore, the averaging times for the
data are not available.
Much higher concentrations of hydrogen sulfide have been measured
near point sources. In California, peak concentrations as high as
0.20 mg/m3 (0.13 ppm) were measured near a pulp and papermill at the
time of its commissioning (California Air Resources Board, 1970).
After operating for several months, levels fell to 0.015 mg/m3
(0.010 ppm) or less. The averaging time was not reported. Near a brick
works in Boom, Belgium, air levels of hydrogen sulfide were monitored
over a 6-month period. During this time, the average 24-h
concentration of hydrogen sulfide was 0.005 mg/m3 (0.003 ppm) with
occasional daily averages in excess of 0.017 mg/m3 (0.011 ppm)
(Institute of Hygiene & Epidemiology, 1978).
A major accidental release of hydrogen sulfide occurred at Poza
Rica, Mexico, in 1950 (McCabe & Clayton, 1952). Although no data could
be collected on environmental levels during this episode, numerous
fatalities occurred indicating that exposure levels were most likely
in excess of 1500-3000 mg/m3 (1000-2000 ppm). Further details of
this episode are given in section 6.3.
In the geothermally active areas in and around the city of
Rotorua, New Zealand, airborne concentrations of hydrogen sulfide are
usually sufficient to cause noticeable odours (Thom & Douglas, 1976).
At one site, for one day, a 1-h mean concentration of up to
2.0 mg/m3 (1.4 ppm) was reported (Thom & Douglas, 1976). Continuous
measurements taken at another site over a period of 5 months showed
that a concentration of 0.08 mg/m3 (0.05 ppm) was exceeded, on
average, 35% of the time. It was also found that there were
considerable seasonal variations in the hydrogen sulfide levels,
reflecting the fluctuating steam-use patterns and also changes in the
dispersive nature of the atmosphere. During the mid-winter months of
the 1978 monitoring period, a concentration of hydrogen sulfide in air
of 0.08 mg/m3 (0.05 ppm) was exceeded more than 55% of the time,
whereas, during warmer months, this concentration was exceeded less
than 20% of the time (Rolfe, 1980).
Also in New Zealand, fine discharge of industrial and domestic
liquid wastes into an inlet near Auckland created conditions in which
hydrogen sulfide levels were sufficient to cause paint blackening and
complaints of offensive odours. Continuous air monitoring was
conducted for 21 months. These data indicated that 40-min average
hydrogen sulfide concentrations in air of from 0.8 to 1.4 mg/m3
(0.5-0.96 ppm) occurred at some time during the worst months of the
year at all the sites monitored (Denmead, 1962).
4.2 Concentrations in Work Places
Under normal operating conditions, concentrations of hydrogen
sulfide in the air in work places are believed to be less than
10-15 mg/m3 (7-10 ppm), the 8-h time weighted average that most
national authorities have set as their occupational exposure standard
(Annex).
It is well known, however, that hazardous exposures to hydrogen
sulfide can occur under accidental circumstances in industries in
which gas streams with a high hydrogen sulfide content exist.
Furthermore, as hydrogen sulfide is slightly heavier than air, it can
accumulate in toxic concentrations in low-lying areas, even when
generated or leaking at very low rates. However, in such cases, the
environmental levels of hydrogen sulfide have usually only been
measured after the accidents in question, or have been determined by
simulation or reenactment. Concentrations that have been reported
range from 150 mg/m3 (100 ppm), in which a worker lost consciousness
while sawing ebonite boards (Brown, 1969), to 18 000 mg/m3
(12 000 ppm) in a case in which a truck driver died while cleaning the
tank of a vehicle used to transport industrial waste (Simson &
Simpson, 1971). In an outdoor setting, 4 workmen lost consciousness
while digging a pit in marshy land in which hydrogen sulfide
concentrations in air of 442-810 mg/m3 (295-540 ppm) were measured 5
days later (Anonymous, 1952). Alexander (1974) reported hydrogen
sulfide concentrations as high as 0.037 mg/litre (24.8 ppm) in a
sewage stabilization pond and 10.0-13.2 mg/m3 (6.7-8.8 ppm) in the
air 15 m from this pond. In a report by Ahlborg (1951) on hydrogen
sulfide poisoning in the Swedish shale oil industry, concentrations of
hydrogen sulfide measured at various locations in the plant ranged
from 30 to 900 mg/m3 (20-600 ppm), though men seldom worked where
the high levels occurred.
More recently, the US National Institute for Occupational Safety
and Health reported that, in viscose rayon churn rooms, spinning
tanks, and drying and storage cells, workers were mainly exposed
during the working day to hydrogen sulfide concentrations of
23 mg/m3 (15 ppm) or less with occasional peaks of 150 mg/m3
(100 ppm) (NIOSH, 1977).
In the USA, it has been estimated that there are 125 000 employees
potentially exposed to hydrogen sulfide (NIOSH, 1977), Table 1 is a
list of occupations in which such exposure can occur ranging according
to occupation from rare exposure to low concentrations, to frequent
exposure to concentrations very near those associated with adverse
health effects.
Table 1. Examples of occupations with potential exposure to
hydrogen sulfidea
Animal fat and oil processors Lithographers
Animal manure removers Lithopone makers
Artificial-flavour makers Livestock farmers
Asphalt storage workers Manhole and trench workers
Barium carbonate makers Metallurgists
Barium salt makers Miners
Blast furnace workers Natural gas production and
Brewery workers processing workers
Bromide-brine workers Painters using polysulflde
Cable splicers caulking compounds
Caisson workers Papermakers
Carbon disulfide makers Petroleum production and
Cellophane makers refinery workers
Chemical laboratory workers, Phosphate purifiers
teachers, students Photo-engravers
Cistern cleaners Pipeline maintenance workers
Citrus root fumigators Pyrite burners
Coal gasification workers Rayon makers
Coke oven workers Refrigerant makers
Copper-ore sulfidizers Rubber and plastics processors
Depilatory makers Septic tank cleaners
Dyemakers Sewage treatment plant workers
Excavators Sewer workers
Felt makers Sheepdippers
Fermentation process workers Silk makers
Fertilizer makers Slaughterhouse workers
Fishing and fish-processing workers Smelting workers
Fur dressers Soapmakers
Geothermal-power drilling and Sugar beet and cane processors
production workers Sulfur spa workers
Gluemakers Sulfur products processors
Gold-ore workers Synthetic-fibre makers
Heavy-metal precipitators Tank gaugers
Heavy-water manufacturers Tannery workers
Hydrochloric acid purifiers Textile printers
Hydrogen sulfide production Thiophene makers
and sales workers Tunnel workers
Landfill workers Well diggers and cleaners
Lead ore sulfidizers Wool pullers
Lead removers
a From: NIOSH (1977).
5. EFFECTS ON EXPERIMENTAL ANIMALS
Very little information is available on the effects of low level
concentrations of hydrogen sulfide gas on experimental animals; most
published data have emphasized the effects of exposure to lethal or
near-lethal concentrations of the gas. According to Evans (1967) and
Smith & Gosselin (1979), the effects of high doses of hydrogen sulfide
and high doses of cyanide are very similar. Both inhibit the enzyme
cytochrome c oxidase [EC 1.9.3.1]. This was demonstrated in studies
using purified preparations of the enzyme (Smith & Gosselin, 1979).
When sodium sulfide at 0.1, 0.25, and 0.32 mmol/kg body weight was
administered intraperitoneally to mice, sulfide was not exhaled
(Susman et al., 1978) suggesting that it was inactivated primarily by
metabolism. In studies on the inhalation of hydrogen sulfide in rats,
cats, rabbits, and dogs, the nervous centres were first excited arid
then paralysed; pupils first contracted and then dilated; blood
pressure was first raised, then lowered; and respiration first
increased and then halted (Evans, 1967; Haggard, 1925). The findings
of Lehmann (1892) in the cat, dog, and rabbit, of Haggard (1925) in
the dog, and of Sayers et al. (1925) in the canary, rat, guineapig,
dog, and goat, are quite consistent: at 150-225 mg/m3 (100-150 ppm),
signs of local irritation of eyes and throat after many hours of
exposure; at 300-450 mg/m3 (200-300 ppm), eye and mucous membrane
irritation after inhalation for 1 h and slight general effects with
prolonged inhalation; at 750-1050 mg/m3 (500-700 ppm), local
irritation and slight systemic symptoms in less than 1 h and possible
death after several hours' exposure; at 1350 mg/m3 (900 ppm), grave
systemic effects within 30 min and death in less than 1 h; at
2250 mg/m3 (1500 ppm), collapse and death within 15-30 min; and at
2700 mg/m3 (1800 ppm), immediate collapse, respiratory paralysis,
and death.
When mice were exposed repeatedly (4 times for 2 h, at 4-day
intervals) to a hydrogen sulfide concentration in air of 150 mg/m3
(100 ppm), the critical inhibition of terminal cytochrome c oxidase
appeared to be cumulative. This effect was accompanied by a cumulative
decrease in cerebral RNA synthesis (Savolainen et al., 1980).
Experimental studies on rabbits indicated that either a single or
repeated exposure for 1.5 h per day (5 consecutive days) to a hydrogen
sulfide concentration in air of 105 mg/m3 (70 ppm) caused
electrocardiographic (ECG) changes (Kósmider et al., 1967).
Though some small differences in susceptibility to hydrogen
sulfide gas were exhibited among the species studied by Sayers et al.,
(1925), canaries being the most sensitive and goats the most
resistant, the interspecies differences were slight. It is agreed
among investigators that the effects of hydrogen sulfide gas on the
nervous system represent the most important aspect of its toxicity
(Haggard, 1925; Evans, 1967). Beck et al. (1979) showed that ethanol
in doses of 0.33-0.66 g/kg significantly shortened the time to loss of
consciousness in rats exposed to a hydrogen sulfide concentration in
air of 1200 mg/m3 (800 ppm) for 30 min. The induction of
methaemoglobinaemia by the injection of sodium nitrite had both
protective and antidotal effects against hydrogen sulfide poisoning in
mice, armadillos, rabbits, and dogs (Smith & Gosselin, 1979).
Water containing a hydrogen sulfide concentration as low as
0.86 mg/litre was toxic to trout after exposure for 24 h (McKee &
Wolf, 1971).
6. EFFECTS ON MAN
Adequate systematic studies of the relationship between hydrogen
sulfide exposure and health status in the general population have not
been carried out. Controlled exposure of human subjects to
concentrations of hydrogen sulfide gas exceeding about 75 mg/m3
(50 ppm) has been deemed to involve excessive risk because of the
possibility of injury to the lungs (Sayers et al., 1925; National
Research Council, USA, 1979). Furthermore, except for studies related
to odour threshold, controlled exposures of human subjects to very low
concentrations of the gas, for example, below 1.5 mg/m3 (1.0 ppm)
have not been reported. Thus, the information presented in this
section has mainly been derived from reports of accidental and
industrial exposures to hydrogen sulfide. A general discussion of the
toxicology of hydrogen sulfide has been included, because a basic
understanding of the subject is necessary for a discussion of the role
of the gas as an industrial and community hazard.
6.1 General Toxicological Considerations
The following observations have been derived from reports of
studies involving man. However, for clarification, some studies on
experimental animals have also been included. In general, both animals
and man respond in a very similar fashion to toxic concentrations of
hydrogen sulfide. It is both an irritant and an asphyxiant gas
(Table 2) that induces local inflammation of the membranes of the
human eye and respiratory tract (Yant, 1930). It has been shown that
eye irritation, the most commonly reported effect of hydrogen sulfide
exposure, can occur after several hours' exposure to concentrations of
16-32 mg/m3 (10.5-21.0 ppm) (Elkins, 1939; Nesswetha, 1969).
However, pulmonary tract irritation is, potentially, a more serious
reaction. When inhaled by dogs, hydrogen sulfide exerted an irritant
action through the entire respiratory tract, though the deeper
structures suffered the greatest damage (Haggard, 1925). Inflammation
of these deeper structures may result in pulmonary oedema.
Exposure to hydrogen sulfide gas did not induce important effects
on the human skin nor was any appreciable absorption through intact
skin observed (Yant, 1930). However, Petrun (1966) reported that when
the skin of rabbits was exposed to hydrogen sulfide at concentrations
of 1050 and 2100 mg/m3 (700 and 1400 ppm), trace amounts of hydrogen
sulfide were found in the exhaled air of the rabbits. No quantitative
information was given.
Hydrogen sulfide gas is rapidly absorbed through the lung. Like
hydrogen cyanide, it is a potent inhibitor of cytochrome c oxidase
that interferes with tissue use (Smith & Gosselin, 1979). As a result,
the oxidative metabolism may slow to the point where tissue metabolic
demands cannot be met. In the central nervous system, the result may
be paralysis of the respiratory centres. Respiratory arrest and death
from asphyxia would be the natural outcome.
In studies on dogs (Haggard, 1925), hydrogen sulfide at
concentrations of 1500-3000 mg/m3 (1000-2000 ppm) initially
stimulated excessively rapid breathing (hyperpnoea), because of a
depletion in the carbon dioxide content of the blood (hypocapnia).
This was followed by a period of respiratory inactivity (apnoea).
Spontaneous respiration may be reestablished, if carbon dioxide
depletion has not progressed beyond the point where prompt
reaccumulation can act as a stimulus to the reestablishment of
respiration. If spontaneous recovery does not occur and artificial
respiration is not applied rapidly, death from asphyxia is the
inevitable result (Haggard, 1925). At about 2250 mg/m3 (1500 ppm),
the sequence of events in the dog was the same, except that the
reaction was more pronounced; at 3000 mg/m3 (2000 ppm) there was
respiratory paralysis after a breath or two and, in Haggard's words,
"the victim falls to the ground as though struck down". When breathing
ceases, generalized convulsions frequently begin. There appears to be
no clear explanation of the cause of this picture of sudden collapse.
According to Haggard (1925), this form of respiratory failure is not
related to the carbon dioxide content of the blood but, rather, to the
directly paralysing effect of hydrogen sulfide on the respiratory
centre. Breathing is never reestablished spontaneously following this
hydrogen sulfide-induced respiratory paralysis. Haggard noted,
however, that because the heart continues to beat for several minutes
after respiration has ceased, death from asphyxia can be prevented if
artificial respiration is begun immediately and is continued until the
hydrogen sulfide concentration in the blood decreases. This decrease
is probably a consequence of metabolic processes, as shown in mice by
Susman et al. (1978) rather than, as once believed, the result of the
pulmonary excretion of the gas.
Smith & Gosselin (1979) have called attention to the confusion
that exists in the literature with regard to the effects of hydrogen
sulfide on haemoglobin. They emphasize that many studies have proved
that neither sulfhaemoglobin nor any other abnormal pigments are
present in sufficient concentrations in the blood of animals or human
subjects, fatally poisoned by hydrogen sulfide.
The characteristic "rotten egg" odour of hydrogen sulfide is an
important aspect of the toxicology of the gas. The threshold of
perception (odour) varies considerably depending on individual
sensitivity. Several authors have reported odour detection thresholds
ranging from 0.0007 mg/m3 to 0.20 mg/m3 (0.0005-0.13 ppm)
(Table 2). Thus, the odour of hydrogen sulfide gas can be a very
sensitive indicator of its presence in low concentrations. However, at
higher concentrations (> 225 mg/m3 (150 ppm)), hydrogen sulfide
exerts a paralysing effect on the olfactory apparatus (Milby, 1962),
thus neutralizing the value of its odour as a warning signal. Poda
(1966) reported that among 42 workers, who were rendered unconscious
from overexposure to hydrogen sulfide, majority did not smell the
characteristic odour of the gas but noted a sickeningly sweet odour,
very briefly, before losing consciousness.
Table 2. Effects of hydrogen sulfide exposure at various concentrations in air
Concentration
Effect Duration of
mg/m3 ppm exposure Reference
Man
Approximate threshold 0.0007-0.2 0.0005-0.13 A few seconds Yant (1930); Ryazanov
for odour to less (1962); Adams & Young
than 1 min (1968); Leonardos et al.
(1969); Lindvall (1970);
Thiele (1979); Winneke
et al. (1979)
Threshold of eye 16-32 10.5-21 6-7 h Elkins (1939)
irritation Nesswetha (1969)
Acute conjuctivitis 75-150 50-100 > 1 h Yant (1930)
(gas eye)
Loss of sense of smell 225-300 150-200 2-15 min Sayers et al. (1925)
Animalsa
Local irritation and 750-1050 500-700 < 1 h Haggard (1925)
slight systemic symptoms;
possible death
after several hours
Table 2. (Con't)
Concentration
Effect Duration of
mg/m3 ppm exposure Reference
Systemic symptoms; 1350 900 < 30 min Haggard (1925)
death in less than 1 h
Death 2250 1500 15-30 min Haggard (1925)
a These observations were made in experimental animals. However, there are no better quantitative
data available concerning man with respect to exposure to hydrogen sulfide at high concentrations.
Hydrogen sulfide intoxication in man has generally been
categorized according to 3 clinical forms, acute, subacute, and
chronic depending on the nature of the predominant clinical signs and
symptoms (National Research Council, USA, 1979). The term "acute
hydrogen sulfide intoxication" has been most often used to describe
systemic poisoning characterized by rapid onset and predominance of
signs and symptoms of nervous system involvement. The term "subacute
intoxication" has been applied to episodes of poisoning in which signs
and symptoms of eye and respiratory tract irritation were most
prominent. "Chronic hydrogen sulfide intoxication" has been applied by
some authors to describe a prolonged state of symptoms resulting from
a single or repeated exposure to concentrations of hydrogen sulfide
that do not produce clear-cut manifestations of either acute or
subacute illness.
In a document prepared by the National Research Council of the
National Academy of Sciences of the USA (National Research Council,
USA, 1979), the observation was made that application of the terms
"acute", "subacute" and "chronic" to hydrogen sulfide exposure was
both imprecise and misleading. However, rather than abandon these
frequently used terms altogether, the authors suggested a series of
clarifying definitions which are quoted in the following paragraphs,
and will henceforth be used in this section.
" Acute intoxication: Effects of a single exposure [seconds-
minutes]a to massive concentrations of hydrogen sulfide that rapidly
produce signs of respiratory distress. Concentrations approximating
1400 mg/m3 (1000 ppm) are usually required to cause acute
intoxication.
Sub-acute intoxication: Effects of continuous exposure [up to
several hours]a to mid-level 140 to 1400 mg/m3 (100 to 1000 ppm)
concentrations of hydrogen sulfide. Eye irritation (gas eye) is the
most commonly reported effect, but pulmonary edema (in the absence of
acute intoxication) has also been noted.
Chronic intoxication: Effects of intermittent exposures to low
to intermediate concentrations 70 to 140 mg/m3 (50 to 100 ppm) of
hydrogen sulfide, characterized by "lingering", largely subjective
manifestations of illness."
It is important to note that these definitions do not include a
consideration of the health consequences to man associated with
prolonged low-level exposure to hydrogen sulfide gas, such as may be
encountered under conditions of general urban air pollution.
A concentration of hydrogen sulfide in drinking water as low as
0.07 µg/litre (0.05 ppm) can affect its taste (Campbell et al., 1958).
a Time factors added by WHO Task Group.
6.2 Occupational Exposure
In certain occupations, workers are intermittently exposed to
concentrations of hydrogen sulfide that are not only malodorous but
can, in some situations, cause severe adverse health effects and even
death. Usually, hydrogen sulfide is encountered in the workplace as an
undesirable by-product of a manufacturing process, notably petroleum
refining, viscose rayon production, sugar beet processing, and tannery
work (Milby, 1962; NIOSH, 1977). In other occupations, for example
cesspool cleaning and work in sewers, exposure to hydrogen sulfide may
occur when the gas is formed as a result of the decomposition of
sulfur-containing organic matter, in the absence of complete
oxidation. Deaths attributed to such exposure occurred in the sewers
of Paris in the 1780s (Mitchell & Davenport, 1924) and still occur
under various circumstances.
Workers in certain occupations risk exposure to naturally
occurring hydrogen sulfide; geothermal energy workers and spa
attendants may be included in this category.
Acute hydrogen sulfide intoxication is a dramatic, often fatal
event. Three men were inadvertently enveloped in a cloud of hydrogen
sulfide gas escaping from a cylinder under high pressure; all fell, as
if struck down, and ceased breathing. Only as a result of prompt
resuscitation by trained onlookers did the men survive, though the two
most seriously affected experienced violent convulsions and did not
recover consciousness for some 30 min. None of the men suffered
important after-effects, and none recalled having noted the
characteristic odour of hydrogen sulfide. The hydrogen sulfide
concentrations to which the men were exposed were estimated to be
about 2800 mg/m3 (2000 ppm) (Milby, 1962). Twelve workmen in a plant
that produced benzyl polysulfide were overcome by hydrogen sulfide
gas, when a pipe used to transfer sodium sulfhydrate ruptured. The
liquid sulfhydrate drained into a nearby sewer, where it reacted with
acid sewage releasing hydrogen sulfide from several sewer openings in
the immediate vicinity. Two of the 12 workmen died, probably as a
result of respiratory arrest; 3 stopped breathing but were
successfully resuscitated; 6 lost consciousness but recovered
spontaneously, and 1 individual developed pulmonary oedema that
responded to therapy (Kleinfeld et al., 1964).
Burnett et al. (1977) reviewed 221 cases of exposure to hydrogen
sulfide associated with the oil, gas, and petrochemical industries in
Canada. The overall mortality was 6%; three-quarters of all victims
experienced a period of unconsciousness and 12% were comatose. A high
proportion of patients had other neurological signs and symptoms,
including altered behaviour patterns, confusion, vertigo, agitation,
or somnolence. Respiratory tract effects were second in frequency only
to neurological manifestations. Forty percent of all cases required
some form of respiratory assistance and 15% of all cases developed
pulmonary oedema. Less severely affected patients complained primarily
of headaches, sore eyes, or gastrointestinal upsets. There were no
recognizable sequelae among the survivors. Data on environmental
exposure levels were not reported.
Nearly fatal cases of acute hydrogen sulfide intoxication
associated with sequelae of varying severity have been reported. A
48-year-old farmer, who collapsed from hydrogen sulfide intoxication
while shovelling manure, continued to have convulsive seizures after
resuscitation. The ECG changes suggested myocardial infarction. The
patient recovered but a slight, persistent dizziness remained
(Kaipainen, 1954). A 46-year-old sewer worker, who was overcome by
hydrogen sulfide in a manhole for 30 min, was cyanotic, suffering
generalized spasms, and required artificial respiration. A week later,
he could move and speak only with great effort; a month afterwards, he
still exhibited neurological deficits. The ECG showed evidence of
small anterolateral infarct and a right bundle branch block. Three
months later, although ambulatory, the patient still suffered from
anginal pain upon exertion (Hurwitz & Taylor, 1954). In a man who had
suffered severe hydrogen sulfide intoxication with collapse and
respiratory failure, ECG evidence of myocardial ischaemia was noted
during the early phase of acute illness but gradually disappeared over
a period of 15 days (Kemper, 1966). Each of these 3 events, in which
sequelae were reported, were characterized by periods of
unconsciousness during which hypoxia of vital issues was likely to
have occurred and may have been the basis for the observed prolonged
effects. Many other instances of sequelae following acute hydrogen
sulfide intoxication have been reported. Most resemble the cases just
mentioned, in which serious poisoning with unconsciousness preceded
the appearance of sequelae.
Ahlborg (1951) described 58 cases of acute hydrogen sulfide
intoxication in Sweden's shale oil industry. There were no fatalities.
Symptoms were generally uniform: sudden fatigue, dizziness, and
intense anxiety followed by unconsciousness with or without
respiratory failure. Several cases of acute poisoning were not
associated with unconsciousness, otherwise symptoms were similar to
those of the other cases. The diagnosis of "sequelae after acute
hydrogen sulfide poisoning" was made in 15 cases. The majority of
these had a history of repeated acute intoxications followed in each
case by neurasthenic problems (fatigue, somnolence, headache, lack of
initiative, irritability, anxiety and poor memory, and decreased
libido), though some developed sequelae following acute intoxication
without an intercurrent episode of unconsciousness. Many stricken
workers developed an increase in sensitivity (aversion) to the odour
of gas of any type, even pure gasoline vapour (Ahlborg, 1951).
Numerous case histories of fatal hydrogen sulfide intoxication
have been reported (Larson et al., 1964; Adelson & Sunshine, 1966;
Simson & Simpson, 1971). Oedema of the lungs and brain are common
post-mortem findings. The presence of detectable concentrations of
hydrogen sulfide in the blood has been reported on several occasions
(Larson et al., 1964; Adelson & Sunshine, 1966) and concentrations in
the blood of fatally poisoned victims have ranged from 1.70 mg to
3.75 mg/litre (McAnalley et al., 1979).
In acute hydrogen sulfide intoxication, cessation of respiration
is an immediate threat to life. Accordingly, the provision of
artificially assisted respiration on an emergency basis is absolutely
critical. There is some question as to whether mouth-to-mouth
resuscitation may create a potential health hazard to the rescuer,
because of the presence of hydrogen sulfide in the expired air or on
the clothing of the victim. Thus, methods of artificial respiration
requiring less direct contact (for example, back-pressure-arm lift)
may be prudent.
6.3 General Population Exposure
There are several reports of episodes of general population
response to air contamination by hydrogen sulfide. Information derived
from these events is consistent with observations reported among
workers occupationally exposed to hydrogen sulfide. Table 2
demonstrates the wide range of odour perception thresholds for
hydrogen sulfide reported by various investigators. In view of the
magnitude of these differences, it is not possible to state with
certainty the concentration at which odour-related complaints can be
expected.
A catastrophic exposure episode involving the release of large
quantities of hydrogen sulfide into a small community was reported by
McCabe & Clayton (1952). This occurred in 1950 in Poza Rica, Mexico, a
city of 22 000 people located about 210 km northeast of Mexico City.
Poza Rica was then the centre of Mexico's leading oil-producing
district and the site of several oil field installations, including a
sulfur-recovery plant. An early morning malfunction of the waste gas
flare resulted in the release of large quantities of unburned hydrogen
sulfide into the atmosphere. The unburned gas, aided by a low-level
temperature inversion and light early morning breezes, was carried to
a residential area adjacent to the plant area. Residents of the area
were overcome while attempting to leave the area and while assisting
stricken neighbours. Within 3 h, 320 persons were hospitalized and 22
died. The most frequent symptom was loss of the sense of smell. More
than half of the patients lost consciousness, many suffered signs and
symptoms of respiratory tract and eye irritation and 9 developed
pulmonary oedema. Four of the 320 victims exhibited neurological
sequelae; 2 experienced neuritis of the acoustic nerve; 1 developed
dysarthria; the fourth patient suffered aggravation of pre-existing
epilepsy. The duration of these neurological sequelae was not
reported.
There have been reports of other episodes of general atmospheric
pollution by hydrogen sulfide evolved from both natural and industrial
sources, but none has been as severe as the Poza Rica incident.
In the geothermal areas of Rotorua, New Zealand, a city of 40 000
people, steam and hot water from approximately 500 active bores are
used to heat houses, buildings, and swimming pools, and to provide
domestic hot water supplies and steam for cooking boxes.
In these areas, accidental fatal cases of acute hydrogen sulfide
intoxication associated with improper ventilation of geothermal steam-
heated dwellings have occasionally been reported. For example, at
least 3 deaths from acute hydrogen sulfide poisoning occurred in 1962.
However, with the introduction of legal requirements regarding the
safe domestic use of the geothermal resources, and possibly also as a
result of increased public awareness of the dangers involved, no
fatalities attributable to hydrogen sulfide have occurred in Rotorua
since 1962 (Thom & Douglas, 1976).
Air pollution monitoring for hydrogen sulfide has been conducted,
during several periods over the past 15 years, at various sites in
Rotorua. However, there has been considerable variation in the results
obtained between the various sites, and furthermore, the
concentrations measured showed considerable seasonal variations. Some
of the results of these measurements are mentioned in section 4.1.
In 1964, the Division of Air Pollution, US Public Health Service,
reported that in the city of Terre Haute, Indiana, biodegradation of
industrial wastes in a 14.5-ha lagoon caused the atmospheric
concentration of hydrogen sulfide to reach a 1-h mean concentration of
0.45 mg/m3 (0.3 ppm). As a result, 81 complaints were registered by
the public, 41 of which were health-related. The most common
complaints were concerned with the perception of a foul odour. The
most common effects were nausea, interruption of sleep, burning of the
eyes, and shortness of breath. Less common manifestations were cough,
headache, and anorexia. Several acute asthma attacks were reported,
but the association of these attacks with the hydrogen sulfide
incident was not clearly established. Though no grave physical
illnesses could be directly related to the air pollution incident, the
investigators stressed that the odours emanating from the lagoon
caused more than a mere nuisance (United States Public Health Service,
1964).
The Poza Rica tragedy provides ample evidence that the accidental
release of hydrogen sulfide into a community can be expected to cause
systemic intoxication of varying severity. The Rotorua experience is
notable in that it emphasizes the fact that the potential for serious,
even fatal, hydrogen sulfide intoxication is present in active
geothermal areas. The more common picture of general population
exposure is exemplified by the Terre Haute incident where an industry-
related source of low-level concentrations of hydrogen sulfide gas
created a health problem which, although not grave, exceeded the level
of mere nuisance.
The potential of long-term, low-level exposure to hydrogen sulfide
to cause pulmonary changes of the type known to be associated with
other irritant gases such as oxides of nitrogen and sulfur has
scarcely been studied. No epidemiological data are available, at
present, upon which to base any sound conclusions.
7. EVALUATION OF HEALTH RISKS TO MAN FROM EXPOSURE TO HYDROGEN SULFIDE
By far the most important recognized toxic effect of hydrogen
sulfide is its ability to induce acute intoxication, characterized by
immediate collapse, frequently accompanied by respiratory arrest and,
without treatment, death. The scientific literature abounds with cases
of this type, most often associated with industrial overexposure.
However, a few cases of acute hydrogen sulfide exposure have been
recorded in the general population as a result of the release of
hydrogen sulfide either from an industrial process or from natural
sources. A second form of injury associated with exposure to hydrogen
sulfide is caused by the irritative action of the gas on the mucous
membranes of the eyes and respiratory tract. Keratoconjunctivitis (gas
eye) and pulmonary oedema are two most serious manifestations of this
local irritative effect. The malodorous property of hydrogen sulfide
gas is well recognized and this characteristic alone is believed by
many to be capable of producing impairment of human health and well-
being.
Most of the information available on human health effects
associated with exposure to various concentrations of hydrogen sulfide
gas has come from observations on accidental and industrial exposures.
With the exception of the Poza Rica catastrophe, information on
general population exposures and associated health effects is sketchy
at best. There is also little information on controlled human exposure
to hydrogen sulfide gas, and, except for data from odour threshold
studies, the information that is available is more than 50 years old.
Although a small amount of information is available on the effects on
experimental animals of high concentrations of hydrogen sulfide gas,
there is virtually no information on the long-term, low-level effects
of the gas on experimental animals. Furthermore, epidemiological data
are lacking concerning the health consequences of long-term, low-level
exposure to hydrogen sulfide, in both the general and industrial
populations.
7.1 Exposure Levels
General population air pollution problems associated with hydrogen
sulfide arise mainly in connexion with malodorous conditions,
traceable to point sources. Such sources can be industrial or, in some
cases, polluted bodies of walter. Peak levels as high as 0.20 mg/m3
(0.13 ppm) have been reported in the air in the neighbourhood of
industrial sources. Hydrogen sulfide is also a common pollutant in
geothermally active areas. At one site is a geothermal area in New
Zealand, where continuous measurements were carried out over a 5-month
period, a level of 0.08 mg/m3 (0.05 ppm) was exceeded, on average,
for 35% of the time.
Concentrations of hydrogen sulfide in the workplace also vary
widely. In the shale oil industry and in viscose rayon production, for
example, maximum levels of exposure during the work day have been
reported to range from 23 to 30 mg/m3 (15-20 ppm). In general,
however, massive accidental exposure to hydrogen sulfide has
constituted the principal hazard of this gas in industrial settings.
In many cases, such exposure has occurred because of equipment
breakage or malfunction. However, because hydrogen sulfide is heavier
than air, it can accumulate in lethal concentrations in low-lying or
enclosed areas. Numerous fatalities have occurred from the slow,
insidious accumulation of the gas in the air in both ambient and
industrial environments.
7.2 Experimental Animal Studies
The toxic effects of hydrogen sulfide gas have not been studied
extensively in experimental animals. However, in studies on a number
of animal species including the mouse, rat, cat, dog, and goat, it has
been shown that the primary target of hydrogen sulfide in high doses
is the nervous system. Collapse, followed by respiratory arrest and
asphyxia resulting from the paralysing effects of high concentrations
of hydrogen sulfide on the respiratory centres of the central nervous
system, is the usual sequence of events leading to death.
Little information is available in the published literature
concerning the effects in experimental animals of long-term, low-level
exposure to hydrogen sulfide.
7.3 Effects of Occupational Exposure
Inadvertent and accidental exposure of human subjects to high
concentrations of hydrogen sulfide has occurred among workers engaged
in petroleum refining, viscose rayon production, sugar beet
processing, and tannery work. Exposure levels have not been precisely
documented in many of these situations. Reported effects range from
the relatively less grave conditions of neurasthenic and
otoneurological symptoms and keratoconjunctivitis to the more serious
effects of pulmonary oedema, respiratory failure, collapse, and even
death. From the available data, it can be estimated that exposure for
seconds or minutes to concentrations of approximately 1400 mg/m3
(1000 ppm) or more would cause acute intoxication, concentrations of
140-1400 mg/m3 (100-1000 ppm) with an exposure time of up to several
hours would produce keratoconjunctivitis and pulmonary oedema, and
intermittent exposures to concentrations of 70-140 mg/m3
(50-100 ppm) could be associated with lingering, largely subjective,
manifestations believed by some to represent chronic intoxication.
Various studies have associated exposure to hydrogen sulfide in
concentrations as low as 16-32 mg/m3 (10.5-21.0 ppm) for several
hours with eye irritation in workers.
Effects of low-level, long-term industrial exposure to hydrogen
sulfide have not been systematically evaluated.
7.4. Effects of General Population Exposure
Several episodes of exposure of the general population to hydrogen
sulfide emanating from a specific source have been investigated and
described. For the most part, these events involved only annoyance
because of the odour or, at worst, minor temporary illness such as
headache, nausea, and sleeplessness. However, as described in section
6.3, on two occasions, general population exposure to hydrogen sulfide
caused grave illness and even death: one at Poza Rica, Mexico, and the
other in and around Rotorua, New Zealand.
Unfortunately, these incidents were not studied using
epidemiological techniques, and it is not possible to establish
exposure-effect relationships from the data.
7.5 Guidelines for the Protection of Public Health
There are two aspects concerning the protection of public health
in relation to hydrogen sulfide exposure: (a) the protection of the
public, and occupational groups in particular, from the toxicological
effects of such exposure; and (b) the protection of the public from
the odour nuisance that can be associated with releases of hydrogen
sulfide.
The odour threshold for hydrogen sulfide has been variously
reported to range from 0.0007 mg to 0.20 mg/m3 (0.0005-0.13 ppm)
(Table 2). Little information is available on the odour detection
limits for ambient hydrogen sulfide either under experimental field
conditions or in general population exposures. The Task Group
considered that a level of 0.007 mg/m3 (0.005 ppm) averaged over
30 min should not produce odour nuisance in most situations. Some
regulatory bodies may wish to adopt longer averaging times with
appropriately adjusted concentration limits.
The best estimates available suggest that eye irritation may occur
in man after several hours' exposure to hydrogen sulfide
concentrations of 16-32 mg/m3 (10.5-21.0 ppm). As occupational
exposure guidelines, the Task Group recommended the adoption of
10 mg/m3 (7 ppm) as a workshift time-weighted average value together
with a short-term exposure limit of 15 mg/m3 (10 ppm). The short-
term limit should be determined as a 10-min or less averaged value.
These limits should prevent eye irritation in workers which represents
the earliest recognized toxic response in man.
Annex tables 1 and 2 contain various national standards or
recommendations for ambient air quality and occupational exposure
limits for hydrogen sulfide. As can be seen, there is considerable
consensus regarding occupational exposure limits and the
recommendations of the Task Group are generally consistent with the
national values. There is less agreement regarding ambient air quality
standards, possibly because of different values placed on the nuisance
value of odours.
REFERENCES
ADAMS, D. F., YOUNG, F. A., & LUHR, R. A. (1968) Evaluation of an odor
perception threshold test facility. Tappi, 51: 62A-67A.
ADELSON, L. & SUNSHINE, I. (1966) Fatal hydrogen sulfide intoxication.
Report of three cases occurring in a sewer. Arch. Pathol.,
81: 375-380.
AHLBORG, G. (1951) Hydrogen sulfide poisoning in shale oil industry.
Am. Med. Assoc. ind. Hyg. occup. Med., 3: 247-266.
AIHA (1965) Hydrogen sulfide. Anal. Abstr., 12: 2207.
ALEXANDER, M. (1974) Microbial formation of environmental pollutants.
Adv. appl. Microbiol., 18: 37-40.
AMERICAN CONFERENCE OF GOVERNMENTAL INDUSTRIAL HYGIENISTS (1972)
Air sampling instruments for evaluation of atmospheric
contaminants, 4th ed., Cincinnati, ACGIH.
ANDERSSON, G., BROSSET, C., & GRENNFELT, P. (1974) The stability of
emitted odorous compounds in the atmosphere. In: Turk, A.,
Johnston, J. W. Jr, & Moulton, D. G., ed. Human response to
environmental odors, New York, Academic Press.
ANONYMOUS (1952) Four workers overcome by hydrogen sulfide when
digging in marshy land. Occup. Health, 12: 39.
BAMESBERGER, W. L. & ADAMS, D. F. (1969) Improvements in the
collection of hydrogen sulfide in cadmium hydroxide suspension.
Environ. Sci. Technol., 3: 258-281.
BAMESBERGER, W. L. & ADAMS, D. F. (1969) Field comparison of the
coulometric, colorimetric, and lead acetate tape analysis methods
for sulfur-containing gases. Tappi, 52: 1302-1306.
BEASLEY, R. W. R. (1963) The eye and hydrogen sulfide. Br. J. ind.
Med., 20: 32-34.
BECK, J. F., CORMIER, F. & DONINI, J. C. (1979) The combined toxicity
of ethanol and hydrogen sulfide. Toxicol. Lett., 3: 311-313.
BECKER, W. J. (1979) On H2S emission and immission measuring
techniques. Staub-Reinhalt. Luft, 39: 169-174.
BERUASHVILI, S. A. (1977) Skin irritant effect of water containing
hydrogen sulfide and results of population studies. In: Annual
Report of the Natadse Institute of Sanitary and Hygiene. Tbilisi,
Vol. XII, pp. 60-63.
BERUASHVILI, S. A. (1979) The long-term effects of the thermal water,
used for the hot water-supply. In: Proceedings of the 4
Conferences of the Young Scientists and Specialists on the
Problem "Environmental Health", Baku, pp. 3-5.
BLACK, M. S., HERBST, R. P., & HITCHCOCK, D. R. (1978) Solid absorbent
preconcentration and gas chromatographic analysis of sulfur gases.
Anal. Chem., 50: 848-851.
BROWN, K. E. (1969) Some toxicological problems in a rubber industry.
Med. J. Aust., 1: 534-538.
BURNETT, W. W., KING, E. G., GRACE, M., & HALL, W. F. (1977) Hydrogen
sulfide poisoning: review of 5 years' experience. Can. Med.
Assoc. J., 117: 1277-1280.
CALIFORNIA AIR RESOURCES BOARD (1970) Ambient air quality standards,
Sacramento, California, California Air Resources Board, pp. 69-75.
CAMPBELL, C. L., DAWES, R. K., DEOLALKAR, S., & MERRITT, M. C. (1958)
Effect of certain chemicals in water on the flavor of brewed
coffee. Food Res., 23: 575-579.
COOPER, R. C., JENKINS, D., & YOUNG, L. Y. (1976) Aquatic
microbiology laboratory manual, Texas, University of Texas.
DENMEAD, C. F. (1962) Air pollution by hydrogen sulfide from a shallow
polluted tidal inlet, Auckland, New Zealand. In: Clean Air
Conference, Sydney, Australia.
ELKINS, H. B. (1939) Toxic fumes. Ind. Med., 8: 426-432.
ESPACH, R. H. (1950) Sources of hydrogen sulfide in Wyoming. Ind.
Eng. Chem., 42: 2235-2237.
EVANS, C. L. (1967) The toxicity of hydrogen sulfide and other
sulfides. Quart. J exp. Physiol., 52: 231-248.
FEDERAL MINISTER FOR HOME AFFAIRS (1974) [Technical instructions for
prevention of air pollution, issued 28 August 1974]. pp. 175
(in German).
FUKUI, S., NAITO, S., KANEKO, M., & KANNO, S. (1967) [Hygienic
chemical studies on public nuisance by injurious gases. X.
Improvement of absorption mixture and examination of the methylene
blue method for determination of hydrogen sulfide.] Hyg. Chem.,
13: 16-21 (in Japanese).
GILARDI, E. F. & MANGANELLI, R. M. (1963) A laboratory study of a lead
acetate-tile method for the quantitative measurement of low
concentrations of hydrogen sulfide. Air Pollut. Control Assoc.,
13: 305-309.
GOLDMAN, F. H., COLEMAN, A. L., ELKINS, H. B., SCHRENK, H. H., &
SMUCKER, C. A. (1940) II. Report of sub-committee on chemical
methods in air analysis. Sampling and sampling devices.
Am. public Health Assoc. Year Book, pp. 92-98.
GOLDMAN, F. H., COLEMAN, A. A., ELKINS, H. B., & SCHRENK, H. H. (1943)
II. Report of sub-committee on chemical methods in air analysis.
Cadmium and hydrogen sulfide. Am. J. public Health, 33: 862-864.
GRANAT, L., RODHE, H., & HALLBERG, R. O. (1976) The global sulfur
cycle. In: Svensson, B. H. & Söderlund, R., ed. Nitrogen,
phosphorus and sulfur -- global cycles, SCOPE report 7. Ecol.
Bull. (Stockholm), 22: 89-134.
HAGGARD, H. W. (1925) The toxicology of hydrogen sulfide. J. ind.
Hyg., 7: 113-121.
HURWITZ, L. J. & TAYLOR, G.I. (1954) Poisoning by sewer gas with
unusual sequelae. Lancet, 1: 1110-1112.
ILO (1971) Tanning, leather finishing. In: Encyclopedia of
Occupational Health and Safety, 2nd ed., Geneva, International
Labour Office, pp. 1381-1382.
ILO (1971-72) Hydrogen sulfide. In: Encyclopedia of Occupational
Health and Safety, 2nd ed., Geneva, International Labour Office,
pp. 697-699.
INSTITUTE OF HYGIENE & EPIDEMIOLOGY (1978) [Air pollution by H2 S
at Boom, Belgium.] Brussels, pp. 1-17 (L.V.M. BO.77) (in Dutch).
ISO (1978) Proposal to the SC3 for the determination of hydrogen
sulfide in air, Geneva, International Organization for
Standardization, 8 pp. (ISO/TC 146/SC3/WG3 N 2E (revised),
1978-06-14).
INTERSOCIETY COMMITTEE (1977 a) Tentative method of analysis for
hydrogen sulfide content of the atmosphere. In: Katz, M., ed.
Methods of air sampling & analysis, 2nd ed., pp. 676-681.
INTERSOCIETY COMMITTEE (1977 b) Tentative method of gas
chromatographic analysis for sulfur-containing gases in the
atmosphere (automatic method with flame photometer detector).
In: Katz, M., ed. Methods of air sampling & analysis, 2nd ed.,
pp. 722-737.
JACOBS, M. B. (1965) Recommended standard method for continuing air
monitoring for hydrogen sulfide. Ultramicrodetermination of
sulfides in the air. J. Air Pollut. Control Assoc., 15: 314-315.
JOHNSON, B. A. (1972) The evaluation of gas detector tube systems:
hydrogen sulfide. Am. Ind. Hyg. Assoc. J., 33: 811-812.
JUNGE, C. E. (1963) Air chemistry and radioactivity, New York,
Academic press, pp. 68-69 (International Geophysics series
Vol. 4).
KAIPAINEN, W. J. (1954) Hydrogen sulfide intoxication. Rapidly
transient changes in the electrocardiogram suggestive of
myocardial infarction. Ann. Med. intern. Fenn., 43: 97-101.
KATZ, M. (1977) Sulfur and its inorganic derivatives in the Canadian
environment, Ottawa, Associate Committee on Scientific Criteria
for Environmental Quality, National Research Council of Canada,
pp. 21-68 (Publ. No. NRCC 15015).
KELLOGG, W. W., CADLE, R. D., ALLEN, E. R., LAZRUS, A. L., & MARTELL,
E. A. (1972) The sulfur cycle. Man's contribution as compared to
natural sources of sulfur compounds in the atmosphere and oceans.
Science, 175: 587-596.
KEMPER, F. D. (1966) A near-fatal case of hydrogen sulfide poisoning.
Can. Med. Assoc. J., 94: 1130-1131.
KLEINFELD, M., GIEL, C., & ROSSO, A. (1964) Acute hydrogen sulfide
intoxication; an unusual source of exposure. Ind. Med. Surg.,
33: 656-660.
KOSMIDER, S., ROGALA, E., & PACHOLEK, A. (1967) Electrocardiographic
and histochemical studies of the heart muscle in acute
experimental hydrogen sulfide poisoning. Arch. Immunol. ther.
Exp., 15: 731-740.
KRASOVITSKAYA, M. L., MALJAROVA, L. K. & ZAPOROZEC, T. S. (1965)
[Contamination of the air in the area of a refinery and
petrochemical plant.] Gig. i Sanit., 4: 103-105 (in Russian).
LARSON, C. P., REBERGER, C. C., & WICKS, M. J. (1964) The purple brain
death. Med. Sci. Law, 4: 200-202.
LAWRENCE BERKELEY LABORATORY (1976) Instrumentation for environmental
monitoring: air pollution. Ambient air monitor, Multi-component
monitoring system for air pollution and SO2 monitor, California,
University of California.
LEHMANN, K. B. (1892) [Experimental studies on the effect on the body
of gases and vapours of technical and hygienic importance, Part V,
hydrogen sulfide.] Arch. Hyg., 14: 135-189 (in German).
LEICHNITZ, K. (1977) Air analyses by means of long-term detector
tubes. Dräger Rev., 40: 9-17.
LEONARDOS, G., KENDALL, D., & BARNARD, N. (1969) Odor threshold
determination of 53 odorant chemicals. J. Air Pollut. Control
Assoc., 19: 91-95.
LEVAGGI, D. A., SIU, W., & FELDSTEIN, M. (1972) Continuous
determination of H2S in the PPB range by a specific colorimetric
method. In: Proceedings of the Technicians International
Congress, N.Y., pp. 65-67.
LINDVALL, T. (1970) On sensory evaluation of odorous air pollutant
intensities. Measurements of odor intensity in the laboratory and
in the field with special reference to effluents of sulfate pulp
factories. Nord. Hyg. Tidskr., 51 (suppl.): 36-39.
MACALUSO, P. (1969) Hydrogen sulfide. In: Mark, H. F., McKetta, J. J.,
& Othmer, D. F., ed. Encyclopedia of chemical technology, 2nd
ed., N.Y., John Wiley & Sons.
McANALLEY, B. H., LOWRY, W. T., OLIVER, R. D., & GARRIOTT, J. C.
(1979) Determination of inorganic sulfide and cyanide in blood
using specific ion electrodes: application to the investigation of
hydrogen sulfide and cyanide poisoning. J. anal. Toxicol.,
3: 111-114.
McCABE, L. C. & CLAYTON, G. D. (1952) Air pollution by hydrogen
sulfide in Poza Rica, Mexico. An evaluation of the incident of
Nov. 24, 1950. Am. Med. Assoc. Arch. ind. Hyg. occup. Med.,
6: 199-213.
McGRAW-HILL ENCYCLOPEDIA OF SCIENCE & TECHNOLOGY (1960) Heavy water,
New York, McGraw-Hill Book Co. Inc., pp. 382-383.
McKEE, J. E. & WOLF, H. W. (ed.) (1971) Water quality criteria, 2nd
ed., California, The Resources Agency of California, State Water
Resources Control Board, 584 pp. (Publ. No. 3-A).
MERCADO, S. G. (1975) Geothermo-electric project of Cerro-Prieto:
contamination and basic protection. In: Proceedings of the Second
UN Symposium on the Development and Use of Geothermal Resources,
San Francisco, CA, pp. 1385-1393.
MILBY, T. H. (1962) Hydrogen sulfide intoxication. Review of the
literature and report of unusual accident resulting in two cases
of nonfatal poisoning. J. occup. Med., 4: 431-437.
MINER, S. (1969) Preliminary air pollution survey of hydrogen
sulfide. A literature review, Raleigh, NC, National air
pollution control adm. (Publ. No. APTD 69-37).
MINSTER, J. T. (1963) Meteorology. Atmospheric hydrogen sulfide
concentrations during the London fog of December 1962. Nature
(Lond.), 199: 474-475.
MITCHELL, C. W. & DAVENPORT, S. J. (1924) Hydrogen sulfide literature.
Public health Rep., 39 (1): 1-13.
NATIONAL RESEARCH COUNCIL, USA (1979) Subcommittee on hydrogen
sulfide. Hydrogen sulfide, Baltimore, University Park Press.
NATUSCH, D. F. S., SEWELL, J. R., & TANNER, R. L. (1974) Determination
of hydrogen sulfide in air -- an assessment of impregnated paper
tape methods. Anal. Chem., 46 (3): 410-415.
NESSWETHA, W. (1969) [Eye lesions caused by sulfur compounds.]
Arbeitsmed. Sozialmed. Arbeitshyg., 4:288-290 (in German).
NEWILL, V. A. (1977) Air quality standards. In: Stern, A. D., ed. Air
pollution, Vol. V, Air quality management, 3rd ed., New York,
Academic Press.
NIOSH (1977) Occupational exposure to hydrogen sulfide, Cincinnati,
OH, National Institute for Occupational Safety and Health (DHEW
(NIOSH) publ. No. 77-158).
NYMAN, H. Th. (1954) Hydrogen sulfide eye inflammation -- treatment
with cortisone. Ind. Med. Surg., 23: 161-162.
PARÉ, J. P. (1966) A new tape reagent for the determination of
hydrogen sulfide in air. J. Air Pollut. Control Assoc.,
16: 325-327.
PEREGUD, Y. A., GERNET, Y. V., & BYKHOOSKAYA, M. S. (1971) Rapid
methods for the determination of noxious substances in the air,
Springfield, VA, US Dept. of Commerce, National Technical
Information Service, (AD 126795).
PETRUN, N. M. (1966) [Penetration of hydrogen sulfide through skin and
its influence on gaseous exchange and energy metabolism.] In:
Pharmacol. Toxicol., 2nd ed., Kiev, Publishing House "Health"
("Zdorovie"), pp. 247-250 (in Russian).
PODA, G. A. (1966) Hydrogen sulfide can be handled safely. Arch.
environ. Health, 12: 795-800.
ROBINSON, E. & ROBBINS, R. C. (1970) Gaseous sulfur pollutants from
urban and natural sources. J. Air Pollut. Control Assoc.,
20: 233-235.
ROLFE, K. A. (1980) The air-pollution aspects of geothermal power
stations. N.Z. energy J., 53: 51-58.
RYAZANOV, V. A. (1962) Sensory physiology as basis for air quality
standards. Arch. environ. Health, 5: 480-494.
SANDERSON, H. P., THOMAS, R., & KATZ, M. (1966) Limitations of the
lead acetate impregnated paper tape method for hydrogen sulfide.
J. Air Pollut. Control Assoc., 16: 328-330.
SAVOLAINEN, H., TENHUNEN, R., ELOVAARA, R., & TOSSAVAINEN, A. (1980)
Cumulative biochemical effects of repeated subclinical hydrogen
sulfide intoxication in mouse brain. Int. Arch. occup. environ.
Health, 46: 87-92.
SAYERS, R. R., SMITH, N. A. C., FIELDNER, A. C., MITCHELL, C. W.,
JONES, G. W., YANT, W. P., STARK, D. D., KATZ, S. H., BLOOMFIELD,
J. J., & JACOBS, W. A. (1925) Investigation of toxic gases from
Mexican and other high-sulfur petroleums and products. Report by
the Dept. of the Interior, Bureau of Mines, to the American
Petroleum Institute, Washington, DC, US Government Printing
Office, pp. 59-80 (Bull. No. 231).
SHAW, J. A. (1940) Rapid determination of hydrogen sulfide and
mercaptan sulfur. In gases and in aqueous solutions. Anal. Chem.,
12: 668-671.
SIMSON, R. E. & SIMPSON, G. R. (1971) Fatal hydrogen sulfide poisoning
associated with industrial waste exposure. Med. J. Aust.,
1: 331-334.
SIU, W., LEVAGGI, D. A., POTTER, L., MARTIN, R., & FELDSTEIN, M.
(1971) Modifications to an H2S tape sampler for increasing
sensitivity and accuracy in H2S sampling. J. Air Pollut.
Control Assoc., 21 (10): 636-638.
SMITH, R. P. & GOSSELIN, R. E. (1979) Hydrogen sulfide poisoning.
J. occup. Med., 21: 93-97.
STEVENS, R. K., MULIK, J. D., O'KEEFFE, A. E., & KROST, K. J. (1971)
Gas chromatography of reactive sulfur gases in air at the parts-
per-billion level. Anal. Chem., 43: 827-831.
STINE, R. J., SLOSBERG, B., & BEACHAM, B. E. (1976) Hydrogen sulfide
intoxication. A case report and discussion of treatment. Ann.
intern. Med., 85 (6): 756-758.
SUSMAN, J. L., HORNIG, J. F., THOMAE, S. C., & SMITH, R. P. (1978)
Pulmonary excretion of hydrogen sulfide, methanethiol, dimethyl
sulfide and dimethyl disulfide in mice. Drug Chem. Toxicol.,
1: 327-338.
THIELE, V. von (1979) [Experimental investigations to determine an
odour threshold value for hydrogen sulfide.] Staub-Reinhalt.
Luft, 39: 159-160 (in German).
THOM, N. G. & DOUGLAS, R. T. (1976) A study of hydrogen sulfide levels
in the geothermal areas of Rotorua, New Zealand. In: Fourth
International Clean Air Congress, Tokyo, pp. 565-568.
THOMPKINS, F. C., Jr & BECKER, J. H. (1976) An evaluation of
portable, direct-reading H2 S meters, Research Report,
Cincinnati, OH, National Institute for Occupational Safety and
Health (DHEW (NIOSH) Publ. No. 77-137).
UNITED STATES PUBLIC HEALTH SERVICE (1941) Hydrogen sulfide: its
toxicity and potential dangers, Cincinnati, OH, US Dept of
Health, Education, and Welfare, Public Health Service, Division of
Air Pollution, pp. 684-693.
UNITED STATES PUBLIC HEALTH SERVICE (1964) The air pollution
situation in Terre Haute, Indiana with special reference to the
hydrogen sulfide incident of May - June, 1964, Cincinnati, OH,
US Dept of Health, Education, and Welfare, Public Health Service
Division of Air Pollution, pp. 1-28.
WEAST, R. C., ed. (1977-78) Physical constants of inorganic compounds.
In: Handbook of chemistry and physics, 58th ed., Cleveland, OH,
USA, CRC Press.
WEST, P. W. (1970) Analytical methods for the study of air pollution.
Pure appl. Chem., 21: 437-447.
WINDHOLZ, M., ed. (1976) Hydrogen sulfide. In: Merck index, 9th ed.,
Rahway, N. J., Merck & Co. Inc., pp. 633-634.
WINNEKE, VON G., KOTALIK, J., KELDENICH, H.-O., & KASTKA, J. (1979)
[The identification of hydrogen sulfide under laboratory and field
conditions.] Staub-Reinhalt. Luft, 39:156-159 (in German).
YANT, W. P. (1930) Hydrogen sulfide in industry, occurrence, effects,
and treatment. Am. J. public Health, 20: 598-608.
Annex
Annex table 1. Ambient air quality standards for hydrogen sulfide from selected countries
Long-term Short-term
Country averaging averaging Reference
mg/m3 ppm time(h) mg/m3 ppm time(min)
Bulgaria 0.008 0.006 24 0.008 0.006 30 Newill (1977)
China -- -- -- 0.01 0.007 20 Official
Communicationd
Czechoslovakia 0.008 0.006 24 0.008 0.006 30 Newill (1977)
German 0.008 0.006 24 0.015 0.011 10--30 Newill (1977)
Democratic
Republic
Federal
Germany, 0.005 0.004 24 0.01 0.007 30 Minister for
Federal Home Affairs
Republic of (1974)
Hungary 0.008a 0.006 24 0.008a 0.008 30 Newill (1977)
Hungary 0.15 0.11 24 0.3 0.21 30 Newill (1977)
Israel 0.045 0.032 24 0.15 0.11 30 Newill (1977)
Philippines -- -- -- 0.3 0.21 30 UNEP/IRPTCe
Poland 0.008b 0.006 24 0.0086 0.008 30 UNEP/IRPTCe
Poland 0.02c 0.014 24 0.06c 0.04 20 UNEP/IRPTCe
Romania 0.01 0.007 24 0.03 0.02 30 Newill (1977)
Spain 0.004 0.003 24 0.01 0.007 30 Newill (1977)
USSR 0.008 0.006 24 0.008 0.006 30 Newill (1977)
Yugoslavia 0.008 0.006 24 0.008 0.006 30 Newill (1977)
a For highly protected and protected areas.
b For especially protected areas, sanatoria, health resorts, sanctuaries, and
national parks.
c For protected areas, towns, and villages.
d Official communication from the Institute of Health, Chinese Academy of Medical
Sciences.
e Private communication.
Note by the WHO Task Group
Ambient Air Quality Standards have been set according to different
criteria in different countries, and these criteria may include, but
are not necessarily limited to the assessment of health effects.
Moreover, the limits themselves may have different meanings such as
the maximum acceptable level or the permissible level over a 10 to
30-min averaging time, etc.
Annex table 2. Occupational exposure standards for hydrogen sulfide
from selected countriesa
Country mg/m3 ppm Standard type
Australia 15 10 8-h TWAb
Belgium 15 10 8-h TWAb
Bulgaria 10 ceiling
China 10 ceiling
Czechoslovakia -- average 10 shiftc TWAb
maximum 20 10-min STELd
Finland 15 10 8-h TWAb
German Democratic Republic -- average 15 8.75-h TWA
short-term 15 30-min STELd
Germany, Federal Republic of 15 10 8-h TWAb
Hungary 10 8-h TWAb
Italy 10 shiftc TWAb
Japan 15 10 shiftc TWAb
Netherlands 15 10 shiftc TWAb
Poland 10 8-h TWAb
Romania -- average 10 shiftTWAb
maximum 15 ceiling
Sweden 15 10 shifte TWAb
Switzerland 15 10 8 to 9-h TWAb
USSR 10 less than 30-min STELd
USA -- occupational standard 20 ceiling except for one
10-min peak less than
50 ppm
ACGIH 15 10 8-h TWAb
21 15 15-min STELd
Yugoslavia 10 7 ceiling
a Abstracted from: ILO (1977).
b TWA = time-weighted average value.
c = a time-weighted value averaged over the entire shift or workday.
d STEL = short-term exposure limit.
hydrogen sulfide (Espach, 1950). Coals can contain sulfur levels of up
to 80 g/kg and, occasionally, conditions arise in which hydrogen
sulfide is formed within such deposits. Thus, special precautions must
be taken in some mining operations as well as in the drilling and
extraction of natural gas and crude oils with significant sulfur
content.
Hydrogen sulfide can also be released by activities surrounding
the development and use of geothermal resources. At the Cerro Prieto
geothermal power generating plant in Baja California, Mexico, for
example, hydrogen sulfide levels are sufficiently high to necessitate
special ventilation to protect electrical systems, and alarms for the
protection of personnel (Mercado, 1975).
During industrial operations, hydrogen sulfide can be formed
whenever elemental sulfur or certain sulfur-containing compounds come
into contact with organic materials at high temperatures. It is
usually produced as an undesirable by-product, though it is also used
as an important reagent or desirable intermediate compound in some
industrial processes such as the manufacture of sulfides, sodium
hydrosulfide, and various organic sulfur compounds. Examples of
processes in which hydrogen sulfide occurs as a by-product include the
production of coke from sulfur-containing coal, the production of
carbon disulfide, the manufacture of viscose rayon in the Kraft
process for producing wood pulp (Macaluso, 1969) and sulfur extraction
by the Frasch process.
In refining sulfur-containing crude oils, about 80%-90% of the
divalent sulfur compounds of hydrogen and carbon are converted to
hydrogen sulfide. Both the hydrogen sulfide produced and that
occurring in other industrial, geothermal, or natural gas streams can
be recovered by one of a number of processes that can be classified as
either absorption-desorption processes or processes involving
oxidation to oxides or to elemental sulfur. The bulk of hydrogen
sulfide recovered in industrial processes is used to produce elemental
sulfur or sulfuric acid (Macaluso, 1969).
Large quantities of hydrogen sulfide are used in the production of
heavy water, which is employed as a moderator in some nuclear power
reactors. The process is based on enrichment of the deuterium content
of water by hydrogen sulfide in a gas/liquid ion exchange system,
followed by separation of heavy water and water by fractional
distillation (McGraw-Hill Encyclopedia of Science and Technology,
1960).
In the tanning industry, hydrogen sulfide is produced in the
process by which hair or wool is removed from the hides. This
typically involves deliming by adding ammonium chloride or ammonium
sulfate followed by pickling with sulfuric acid, and takes place in
large rotating drums. The gases evolved, including hydrogen sulfide,
are released from the drums on opening the hatches either to add
chemicals or to unload the treated hides, and also from the waste
waters (ILO, 1971).
As in the natural environment, hydrogen sulfide can be generated
by bacterial action in industrial or community settings in malodorous
and sometimes dangerous amounts.
In some countries, such as India and Sri Lanka, hydrogen sulfide
is produced in the process by which coconut fibres are separated from
the husk. This procedure involves the decomposition of the husks in
shallow ponds. The hydrogen sulfide is produced as a result of
microbiological decay processes.
4. ENVIRONMENTAL LEVELS AND EXPOSURE
4.1 Concentrations in Outdoor Air
There are few published data on either natural background or urban
air levels of hydrogen sulfide. Robinson & Robbins (1970) estimated
the average ambient air level of hydrogen sulfide to be 0.0003 mg/m3
(0.0002 ppm). This estimate supports the data of Minster (1963) who,
when sampling over a 2´-year period in northwest London, reported that
air levels of hydrogen sulfide were generally below 0.00015 mg/m3
(0.0001 ppm), under clear, fresh conditions. Minster (1963) also
reported that average summer levels ranged from 0.00015 to 0.0007
mg/m3 (0.0001-0.0005 ppm) and average winter levels from 0.0007 to
0.0015 mg/m3 (0.0005-0.001 ppm). Data collected from this same
station during the London fog of December 1962 indicated a hydrogen
sulfide concentration of up to 0.046 mg/m3 (0.033 ppm) on 6 December
during heavy fog (each sampling time was 32 min).
Measurements summarized by the US National Air Pollution Control
Administration show concentrations ranging from below 0.001 mg/m3 to
0.006 mg/m3 (0.0007 to 0.0042 ppm) at various urban locations in the
USA in the period 1951-64 (Miner, 1969). However, as sensitive and
standardized methods of sampling and analysis for hydrogen sulfide
were lacking during this period, there is some doubt about the
reliability of these data. Furthermore, the averaging times for the
data are not available.
Much higher concentrations of hydrogen sulfide have been measured
near point sources. In California, peak concentrations as high as
0.20 mg/m3 (0.13 ppm) were measured near a pulp and papermill at the
time of its commissioning (California Air Resources Board, 1970).
After operating for several months, levels fell to 0.015 mg/m3
(0.010 ppm) or less. The averaging time was not reported. Near a brick
works in Boom, Belgium, air levels of hydrogen sulfide were monitored
over a 6-month period. During this time, the average 24-h
concentration of hydrogen sulfide was 0.005 mg/m3 (0.003 ppm) with
occasional daily averages in excess of 0.017 mg/m3 (0.011 ppm)
(Institute of Hygiene & Epidemiology, 1978).
A major accidental release of hydrogen sulfide occurred at Poza
Rica, Mexico, in 1950 (McCabe & Clayton, 1952). Although no data could
be collected on environmental levels during this episode, numerous
fatalities occurred indicating that exposure levels were most likely
in excess of 1500-3000 mg/m3 (1000-2000 ppm). Further details of
this episode are given in section 6.3.
In the geothermally active areas in and around the city of
Rotorua, New Zealand, airborne concentrations of hydrogen sulfide are
usually sufficient to cause noticeable odours (Thom & Douglas, 1976).
At one site, for one day, a 1-h mean concentration of up to
2.0 mg/m3 (1.4 ppm) was reported (Thom & Douglas, 1976). Continuous
measurements taken at another site over a period of 5 months showed
that a concentration of 0.08 mg/m3 (0.05 ppm) was exceeded, on
average, 35% of the time. It was also found that there were
considerable seasonal variations in the hydrogen sulfide levels,
reflecting the fluctuating steam-use patterns and also changes in the
dispersive nature of the atmosphere. During the mid-winter months of
the 1978 monitoring period, a concentration of hydrogen sulfide in air
of 0.08 mg/m3 (0.05 ppm) was exceeded more than 55% of the time,
whereas, during warmer months, this concentration was exceeded less
than 20% of the time (Rolfe, 1980).
Also in New Zealand, fine discharge of industrial and domestic
liquid wastes into an inlet near Auckland created conditions in which
hydrogen sulfide levels were sufficient to cause paint blackening and
complaints of offensive odours. Continuous air monitoring was
conducted for 21 months. These data indicated that 40-min average
hydrogen sulfide concentrations in air of from 0.8 to 1.4 mg/m3
(0.5-0.96 ppm) occurred at some time during the worst months of the
year at all the sites monitored (Denmead, 1962).
4.2 Concentrations in Work Places
Under normal operating conditions, concentrations of hydrogen
sulfide in the air in work places are believed to be less than
10-15 mg/m3 (7-10 ppm), the 8-h time weighted average that most
national authorities have set as their occupational exposure standard
(Annex).
It is well known, however, that hazardous exposures to hydrogen
sulfide can occur under accidental circumstances in industries in
which gas streams with a high hydrogen sulfide content exist.
Furthermore, as hydrogen sulfide is slightly heavier than air, it can
accumulate in toxic concentrations in low-lying areas, even when
generated or leaking at very low rates. However, in such cases, the
environmental levels of hydrogen sulfide have usually only been
measured after the accidents in question, or have been determined by
simulation or reenactment. Concentrations that have been reported
range from 150 mg/m3 (100 ppm), in which a worker lost consciousness
while sawing ebonite boards (Brown, 1969), to 18 000 mg/m3
(12 000 ppm) in a case in which a truck driver died while cleaning the
tank of a vehicle used to transport industrial waste (Simson &
Simpson, 1971). In an outdoor setting, 4 workmen lost consciousness
while digging a pit in marshy land in which hydrogen sulfide
concentrations in air of 442-810 mg/m3 (295-540 ppm) were measured 5
days later (Anonymous, 1952). Alexander (1974) reported hydrogen
sulfide concentrations as high as 0.037 mg/litre (24.8 ppm) in a
sewage stabilization pond and 10.0-13.2 mg/m3 (6.7-8.8 ppm) in the
air 15 m from this pond. In a report by Ahlborg (1951) on hydrogen
sulfide poisoning in the Swedish shale oil industry, concentrations of
hydrogen sulfide measured at various locations in the plant ranged
from 30 to 900 mg/m3 (20-600 ppm), though men seldom worked where
the high levels occurred.
More recently, the US National Institute for Occupational Safety
and Health reported that, in viscose rayon churn rooms, spinning
tanks, and drying and storage cells, workers were mainly exposed
during the working day to hydrogen sulfide concentrations of
23 mg/m3 (15 ppm) or less with occasional peaks of 150 mg/m3
(100 ppm) (NIOSH, 1977).
In the USA, it has been estimated that there are 125 000 employees
potentially exposed to hydrogen sulfide (NIOSH, 1977), Table 1 is a
list of occupations in which such exposure can occur ranging according
to occupation from rare exposure to low concentrations, to frequent
exposure to concentrations very near those associated with adverse
health effects.
Table 1. Examples of occupations with potential exposure to
hydrogen sulfidea
Animal fat and oil processors Lithographers
Animal manure removers Lithopone makers
Artificial-flavour makers Livestock farmers
Asphalt storage workers Manhole and trench workers
Barium carbonate makers Metallurgists
Barium salt makers Miners
Blast furnace workers Natural gas production and
Brewery workers processing workers
Bromide-brine workers Painters using polysulflde
Cable splicers caulking compounds
Caisson workers Papermakers
Carbon disulfide makers Petroleum production and
Cellophane makers refinery workers
Chemical laboratory workers, Phosphate purifiers
teachers, students Photo-engravers
Cistern cleaners Pipeline maintenance workers
Citrus root fumigators Pyrite burners
Coal gasification workers Rayon makers
Coke oven workers Refrigerant makers
Copper-ore sulfidizers Rubber and plastics processors
Depilatory makers Septic tank cleaners
Dyemakers Sewage treatment plant workers
Excavators Sewer workers
Felt makers Sheepdippers
Fermentation process workers Silk makers
Fertilizer makers Slaughterhouse workers
Fishing and fish-processing workers Smelting workers
Fur dressers Soapmakers
Geothermal-power drilling and Sugar beet and cane processors
production workers Sulfur spa workers
Gluemakers Sulfur products processors
Gold-ore workers Synthetic-fibre makers
Heavy-metal precipitators Tank gaugers
Heavy-water manufacturers Tannery workers
Hydrochloric acid purifiers Textile printers
Hydrogen sulfide production Thiophene makers
and sales workers Tunnel workers
Landfill workers Well diggers and cleaners
Lead ore sulfidizers Wool pullers
Lead removers
a From: NIOSH (1977).
5. EFFECTS ON EXPERIMENTAL ANIMALS
Very little information is available on the effects of low level
concentrations of hydrogen sulfide gas on experimental animals; most
published data have emphasized the effects of exposure to lethal or
near-lethal concentrations of the gas. According to Evans (1967) and
Smith & Gosselin (1979), the effects of high doses of hydrogen sulfide
and high doses of cyanide are very similar. Both inhibit the enzyme
cytochrome c oxidase [EC 1.9.3.1]. This was demonstrated in studies
using purified preparations of the enzyme (Smith & Gosselin, 1979).
When sodium sulfide at 0.1, 0.25, and 0.32 mmol/kg body weight was
administered intraperitoneally to mice, sulfide was not exhaled
(Susman et al., 1978) suggesting that it was inactivated primarily by
metabolism. In studies on the inhalation of hydrogen sulfide in rats,
cats, rabbits, and dogs, the nervous centres were first excited arid
then paralysed; pupils first contracted and then dilated; blood
pressure was first raised, then lowered; and respiration first
increased and then halted (Evans, 1967; Haggard, 1925). The findings
of Lehmann (1892) in the cat, dog, and rabbit, of Haggard (1925) in
the dog, and of Sayers et al. (1925) in the canary, rat, guineapig,
dog, and goat, are quite consistent: at 150-225 mg/m3 (100-150 ppm),
signs of local irritation of eyes and throat after many hours of
exposure; at 300-450 mg/m3 (200-300 ppm), eye and mucous membrane
irritation after inhalation for 1 h and slight general effects with
prolonged inhalation; at 750-1050 mg/m3 (500-700 ppm), local
irritation and slight systemic symptoms in less than 1 h and possible
death after several hours' exposure; at 1350 mg/m3 (900 ppm), grave
systemic effects within 30 min and death in less than 1 h; at
2250 mg/m3 (1500 ppm), collapse and death within 15-30 min; and at
2700 mg/m3 (1800 ppm), immediate collapse, respiratory paralysis,
and death.
When mice were exposed repeatedly (4 times for 2 h, at 4-day
intervals) to a hydrogen sulfide concentration in air of 150 mg/m3
(100 ppm), the critical inhibition of terminal cytochrome c oxidase
appeared to be cumulative. This effect was accompanied by a cumulative
decrease in cerebral RNA synthesis (Savolainen et al., 1980).
Experimental studies on rabbits indicated that either a single or
repeated exposure for 1.5 h per day (5 consecutive days) to a hydrogen
sulfide concentration in air of 105 mg/m3 (70 ppm) caused
electrocardiographic (ECG) changes (Kósmider et al., 1967).
Though some small differences in susceptibility to hydrogen
sulfide gas were exhibited among the species studied by Sayers et al.,
(1925), canaries being the most sensitive and goats the most
resistant, the interspecies differences were slight. It is agreed
among investigators that the effects of hydrogen sulfide gas on the
nervous system represent the most important aspect of its toxicity
(Haggard, 1925; Evans, 1967). Beck et al. (1979) showed that ethanol
in doses of 0.33-0.66 g/kg significantly shortened the time to loss of
consciousness in rats exposed to a hydrogen sulfide concentration in
air of 1200 mg/m3 (800 ppm) for 30 min. The induction of
methaemoglobinaemia by the injection of sodium nitrite had both
protective and antidotal effects against hydrogen sulfide poisoning in
mice, armadillos, rabbits, and dogs (Smith & Gosselin, 1979).
Water containing a hydrogen sulfide concentration as low as
0.86 mg/litre was toxic to trout after exposure for 24 h (McKee &
Wolf, 1971).
6. EFFECTS ON MAN
Adequate systematic studies of the relationship between hydrogen
sulfide exposure and health status in the general population have not
been carried out. Controlled exposure of human subjects to
concentrations of hydrogen sulfide gas exceeding about 75 mg/m3
(50 ppm) has been deemed to involve excessive risk because of the
possibility of injury to the lungs (Sayers et al., 1925; National
Research Council, USA, 1979). Furthermore, except for studies related
to odour threshold, controlled exposures of human subjects to very low
concentrations of the gas, for example, below 1.5 mg/m3 (1.0 ppm)
have not been reported. Thus, the information presented in this
section has mainly been derived from reports of accidental and
industrial exposures to hydrogen sulfide. A general discussion of the
toxicology of hydrogen sulfide has been included, because a basic
understanding of the subject is necessary for a discussion of the role
of the gas as an industrial and community hazard.
6.1 General Toxicological Considerations
The following observations have been derived from reports of
studies involving man. However, for clarification, some studies on
experimental animals have also been included. In general, both animals
and man respond in a very similar fashion to toxic concentrations of
hydrogen sulfide. It is both an irritant and an asphyxiant gas
(Table 2) that induces local inflammation of the membranes of the
human eye and respiratory tract (Yant, 1930). It has been shown that
eye irritation, the most commonly reported effect of hydrogen sulfide
exposure, can occur after several hours' exposure to concentrations of
16-32 mg/m3 (10.5-21.0 ppm) (Elkins, 1939; Nesswetha, 1969).
However, pulmonary tract irritation is, potentially, a more serious
reaction. When inhaled by dogs, hydrogen sulfide exerted an irritant
action through the entire respiratory tract, though the deeper
structures suffered the greatest damage (Haggard, 1925). Inflammation
of these deeper structures may result in pulmonary oedema.
Exposure to hydrogen sulfide gas did not induce important effects
on the human skin nor was any appreciable absorption through intact
skin observed (Yant, 1930). However, Petrun (1966) reported that when
the skin of rabbits was exposed to hydrogen sulfide at concentrations
of 1050 and 2100 mg/m3 (700 and 1400 ppm), trace amounts of hydrogen
sulfide were found in the exhaled air of the rabbits. No quantitative
information was given.
Hydrogen sulfide gas is rapidly absorbed through the lung. Like
hydrogen cyanide, it is a potent inhibitor of cytochrome c oxidase
that interferes with tissue use (Smith & Gosselin, 1979). As a result,
the oxidative metabolism may slow to the point where tissue metabolic
demands cannot be met. In the central nervous system, the result may
be paralysis of the respiratory centres. Respiratory arrest and death
from asphyxia would be the natural outcome.
In studies on dogs (Haggard, 1925), hydrogen sulfide at
concentrations of 1500-3000 mg/m3 (1000-2000 ppm) initially
stimulated excessively rapid breathing (hyperpnoea), because of a
depletion in the carbon dioxide content of the blood (hypocapnia).
This was followed by a period of respiratory inactivity (apnoea).
Spontaneous respiration may be reestablished, if carbon dioxide
depletion has not progressed beyond the point where prompt
reaccumulation can act as a stimulus to the reestablishment of
respiration. If spontaneous recovery does not occur and artificial
respiration is not applied rapidly, death from asphyxia is the
inevitable result (Haggard, 1925). At about 2250 mg/m3 (1500 ppm),
the sequence of events in the dog was the same, except that the
reaction was more pronounced; at 3000 mg/m3 (2000 ppm) there was
respiratory paralysis after a breath or two and, in Haggard's words,
"the victim falls to the ground as though struck down". When breathing
ceases, generalized convulsions frequently begin. There appears to be
no clear explanation of the cause of this picture of sudden collapse.
According to Haggard (1925), this form of respiratory failure is not
related to the carbon dioxide content of the blood but, rather, to the
directly paralysing effect of hydrogen sulfide on the respiratory
centre. Breathing is never reestablished spontaneously following this
hydrogen sulfide-induced respiratory paralysis. Haggard noted,
however, that because the heart continues to beat for several minutes
after respiration has ceased, death from asphyxia can be prevented if
artificial respiration is begun immediately and is continued until the
hydrogen sulfide concentration in the blood decreases. This decrease
is probably a consequence of metabolic processes, as shown in mice by
Susman et al. (1978) rather than, as once believed, the result of the
pulmonary excretion of the gas.
Smith & Gosselin (1979) have called attention to the confusion
that exists in the literature with regard to the effects of hydrogen
sulfide on haemoglobin. They emphasize that many studies have proved
that neither sulfhaemoglobin nor any other abnormal pigments are
present in sufficient concentrations in the blood of animals or human
subjects, fatally poisoned by hydrogen sulfide.
The characteristic "rotten egg" odour of hydrogen sulfide is an
important aspect of the toxicology of the gas. The threshold of
perception (odour) varies considerably depending on individual
sensitivity. Several authors have reported odour detection thresholds
ranging from 0.0007 mg/m3 to 0.20 mg/m3 (0.0005-0.13 ppm)
(Table 2). Thus, the odour of hydrogen sulfide gas can be a very
sensitive indicator of its presence in low concentrations. However, at
higher concentrations (> 225 mg/m3 (150 ppm)), hydrogen sulfide
exerts a paralysing effect on the olfactory apparatus (Milby, 1962),
thus neutralizing the value of its odour as a warning signal. Poda
(1966) reported that among 42 workers, who were rendered unconscious
from overexposure to hydrogen sulfide, majority did not smell the
characteristic odour of the gas but noted a sickeningly sweet odour,
very briefly, before losing consciousness.
Table 2. Effects of hydrogen sulfide exposure at various concentrations in air
Concentration
Effect Duration of
mg/m3 ppm exposure Reference
Man
Approximate threshold 0.0007-0.2 0.0005-0.13 A few seconds Yant (1930); Ryazanov
for odour to less (1962); Adams & Young
than 1 min (1968); Leonardos et al.
(1969); Lindvall (1970);
Thiele (1979); Winneke
et al. (1979)
Threshold of eye 16-32 10.5-21 6-7 h Elkins (1939)
irritation Nesswetha (1969)
Acute conjuctivitis 75-150 50-100 > 1 h Yant (1930)
(gas eye)
Loss of sense of smell 225-300 150-200 2-15 min Sayers et al. (1925)
Animalsa
Local irritation and 750-1050 500-700 < 1 h Haggard (1925)
slight systemic symptoms;
possible death
after several hours
Table 2. (Con't)
Concentration
Effect Duration of
mg/m3 ppm exposure Reference
Systemic symptoms; 1350 900 < 30 min Haggard (1925)
death in less than 1 h
Death 2250 1500 15-30 min Haggard (1925)
a These observations were made in experimental animals. However, there are no better quantitative
data available concerning man with respect to exposure to hydrogen sulfide at high concentrations.
Hydrogen sulfide intoxication in man has generally been
categorized according to 3 clinical forms, acute, subacute, and
chronic depending on the nature of the predominant clinical signs and
symptoms (National Research Council, USA, 1979). The term "acute
hydrogen sulfide intoxication" has been most often used to describe
systemic poisoning characterized by rapid onset and predominance of
signs and symptoms of nervous system involvement. The term "subacute
intoxication" has been applied to episodes of poisoning in which signs
and symptoms of eye and respiratory tract irritation were most
prominent. "Chronic hydrogen sulfide intoxication" has been applied by
some authors to describe a prolonged state of symptoms resulting from
a single or repeated exposure to concentrations of hydrogen sulfide
that do not produce clear-cut manifestations of either acute or
subacute illness.
In a document prepared by the National Research Council of the
National Academy of Sciences of the USA (National Research Council,
USA, 1979), the observation was made that application of the terms
"acute", "subacute" and "chronic" to hydrogen sulfide exposure was
both imprecise and misleading. However, rather than abandon these
frequently used terms altogether, the authors suggested a series of
clarifying definitions which are quoted in the following paragraphs,
and will henceforth be used in this section.
" Acute intoxication: Effects of a single exposure [seconds-
minutes]a to massive concentrations of hydrogen sulfide that rapidly
produce signs of respiratory distress. Concentrations approximating
1400 mg/m3 (1000 ppm) are usually required to cause acute
intoxication.
Sub-acute intoxication: Effects of continuous exposure [up to
several hours]a to mid-level 140 to 1400 mg/m3 (100 to 1000 ppm)
concentrations of hydrogen sulfide. Eye irritation (gas eye) is the
most commonly reported effect, but pulmonary edema (in the absence of
acute intoxication) has also been noted.
Chronic intoxication: Effects of intermittent exposures to low
to intermediate concentrations 70 to 140 mg/m3 (50 to 100 ppm) of
hydrogen sulfide, characterized by "lingering", largely subjective
manifestations of illness."
It is important to note that these definitions do not include a
consideration of the health consequences to man associated with
prolonged low-level exposure to hydrogen sulfide gas, such as may be
encountered under conditions of general urban air pollution.
A concentration of hydrogen sulfide in drinking water as low as
0.07 µg/litre (0.05 ppm) can affect its taste (Campbell et al., 1958).
a Time factors added by WHO Task Group.
6.2 Occupational Exposure
In certain occupations, workers are intermittently exposed to
concentrations of hydrogen sulfide that are not only malodorous but
can, in some situations, cause severe adverse health effects and even
death. Usually, hydrogen sulfide is encountered in the workplace as an
undesirable by-product of a manufacturing process, notably petroleum
refining, viscose rayon production, sugar beet processing, and tannery
work (Milby, 1962; NIOSH, 1977). In other occupations, for example
cesspool cleaning and work in sewers, exposure to hydrogen sulfide may
occur when the gas is formed as a result of the decomposition of
sulfur-containing organic matter, in the absence of complete
oxidation. Deaths attributed to such exposure occurred in the sewers
of Paris in the 1780s (Mitchell & Davenport, 1924) and still occur
under various circumstances.
Workers in certain occupations risk exposure to naturally
occurring hydrogen sulfide; geothermal energy workers and spa
attendants may be included in this category.
Acute hydrogen sulfide intoxication is a dramatic, often fatal
event. Three men were inadvertently enveloped in a cloud of hydrogen
sulfide gas escaping from a cylinder under high pressure; all fell, as
if struck down, and ceased breathing. Only as a result of prompt
resuscitation by trained onlookers did the men survive, though the two
most seriously affected experienced violent convulsions and did not
recover consciousness for some 30 min. None of the men suffered
important after-effects, and none recalled having noted the
characteristic odour of hydrogen sulfide. The hydrogen sulfide
concentrations to which the men were exposed were estimated to be
about 2800 mg/m3 (2000 ppm) (Milby, 1962). Twelve workmen in a plant
that produced benzyl polysulfide were overcome by hydrogen sulfide
gas, when a pipe used to transfer sodium sulfhydrate ruptured. The
liquid sulfhydrate drained into a nearby sewer, where it reacted with
acid sewage releasing hydrogen sulfide from several sewer openings in
the immediate vicinity. Two of the 12 workmen died, probably as a
result of respiratory arrest; 3 stopped breathing but were
successfully resuscitated; 6 lost consciousness but recovered
spontaneously, and 1 individual developed pulmonary oedema that
responded to therapy (Kleinfeld et al., 1964).
Burnett et al. (1977) reviewed 221 cases of exposure to hydrogen
sulfide associated with the oil, gas, and petrochemical industries in
Canada. The overall mortality was 6%; three-quarters of all victims
experienced a period of unconsciousness and 12% were comatose. A high
proportion of patients had other neurological signs and symptoms,
including altered behaviour patterns, confusion, vertigo, agitation,
or somnolence. Respiratory tract effects were second in frequency only
to neurological manifestations. Forty percent of all cases required
some form of respiratory assistance and 15% of all cases developed
pulmonary oedema. Less severely affected patients complained primarily
of headaches, sore eyes, or gastrointestinal upsets. There were no
recognizable sequelae among the survivors. Data on environmental
exposure levels were not reported.
Nearly fatal cases of acute hydrogen sulfide intoxication
associated with sequelae of varying severity have been reported. A
48-year-old farmer, who collapsed from hydrogen sulfide intoxication
while shovelling manure, continued to have convulsive seizures after
resuscitation. The ECG changes suggested myocardial infarction. The
patient recovered but a slight, persistent dizziness remained
(Kaipainen, 1954). A 46-year-old sewer worker, who was overcome by
hydrogen sulfide in a manhole for 30 min, was cyanotic, suffering
generalized spasms, and required artificial respiration. A week later,
he could move and speak only with great effort; a month afterwards, he
still exhibited neurological deficits. The ECG showed evidence of
small anterolateral infarct and a right bundle branch block. Three
months later, although ambulatory, the patient still suffered from
anginal pain upon exertion (Hurwitz & Taylor, 1954). In a man who had
suffered severe hydrogen sulfide intoxication with collapse and
respiratory failure, ECG evidence of myocardial ischaemia was noted
during the early phase of acute illness but gradually disappeared over
a period of 15 days (Kemper, 1966). Each of these 3 events, in which
sequelae were reported, were characterized by periods of
unconsciousness during which hypoxia of vital issues was likely to
have occurred and may have been the basis for the observed prolonged
effects. Many other instances of sequelae following acute hydrogen
sulfide intoxication have been reported. Most resemble the cases just
mentioned, in which serious poisoning with unconsciousness preceded
the appearance of sequelae.
Ahlborg (1951) described 58 cases of acute hydrogen sulfide
intoxication in Sweden's shale oil industry. There were no fatalities.
Symptoms were generally uniform: sudden fatigue, dizziness, and
intense anxiety followed by unconsciousness with or without
respiratory failure. Several cases of acute poisoning were not
associated with unconsciousness, otherwise symptoms were similar to
those of the other cases. The diagnosis of "sequelae after acute
hydrogen sulfide poisoning" was made in 15 cases. The majority of
these had a history of repeated acute intoxications followed in each
case by neurasthenic problems (fatigue, somnolence, headache, lack of
initiative, irritability, anxiety and poor memory, and decreased
libido), though some developed sequelae following acute intoxication
without an intercurrent episode of unconsciousness. Many stricken
workers developed an increase in sensitivity (aversion) to the odour
of gas of any type, even pure gasoline vapour (Ahlborg, 1951).
Numerous case histories of fatal hydrogen sulfide intoxication
have been reported (Larson et al., 1964; Adelson & Sunshine, 1966;
Simson & Simpson, 1971). Oedema of the lungs and brain are common
post-mortem findings. The presence of detectable concentrations of
hydrogen sulfide in the blood has been reported on several occasions
(Larson et al., 1964; Adelson & Sunshine, 1966) and concentrations in
the blood of fatally poisoned victims have ranged from 1.70 mg to
3.75 mg/litre (McAnalley et al., 1979).
In acute hydrogen sulfide intoxication, cessation of respiration
is an immediate threat to life. Accordingly, the provision of
artificially assisted respiration on an emergency basis is absolutely
critical. There is some question as to whether mouth-to-mouth
resuscitation may create a potential health hazard to the rescuer,
because of the presence of hydrogen sulfide in the expired air or on
the clothing of the victim. Thus, methods of artificial respiration
requiring less direct contact (for example, back-pressure-arm lift)
may be prudent.
6.3 General Population Exposure
There are several reports of episodes of general population
response to air contamination by hydrogen sulfide. Information derived
from these events is consistent with observations reported among
workers occupationally exposed to hydrogen sulfide. Table 2
demonstrates the wide range of odour perception thresholds for
hydrogen sulfide reported by various investigators. In view of the
magnitude of these differences, it is not possible to state with
certainty the concentration at which odour-related complaints can be
expected.
A catastrophic exposure episode involving the release of large
quantities of hydrogen sulfide into a small community was reported by
McCabe & Clayton (1952). This occurred in 1950 in Poza Rica, Mexico, a
city of 22 000 people located about 210 km northeast of Mexico City.
Poza Rica was then the centre of Mexico's leading oil-producing
district and the site of several oil field installations, including a
sulfur-recovery plant. An early morning malfunction of the waste gas
flare resulted in the release of large quantities of unburned hydrogen
sulfide into the atmosphere. The unburned gas, aided by a low-level
temperature inversion and light early morning breezes, was carried to
a residential area adjacent to the plant area. Residents of the area
were overcome while attempting to leave the area and while assisting
stricken neighbours. Within 3 h, 320 persons were hospitalized and 22
died. The most frequent symptom was loss of the sense of smell. More
than half of the patients lost consciousness, many suffered signs and
symptoms of respiratory tract and eye irritation and 9 developed
pulmonary oedema. Four of the 320 victims exhibited neurological
sequelae; 2 experienced neuritis of the acoustic nerve; 1 developed
dysarthria; the fourth patient suffered aggravation of pre-existing
epilepsy. The duration of these neurological sequelae was not
reported.
There have been reports of other episodes of general atmospheric
pollution by hydrogen sulfide evolved from both natural and industrial
sources, but none has been as severe as the Poza Rica incident.
In the geothermal areas of Rotorua, New Zealand, a city of 40 000
people, steam and hot water from approximately 500 active bores are
used to heat houses, buildings, and swimming pools, and to provide
domestic hot water supplies and steam for cooking boxes.
In these areas, accidental fatal cases of acute hydrogen sulfide
intoxication associated with improper ventilation of geothermal steam-
heated dwellings have occasionally been reported. For example, at
least 3 deaths from acute hydrogen sulfide poisoning occurred in 1962.
However, with the introduction of legal requirements regarding the
safe domestic use of the geothermal resources, and possibly also as a
result of increased public awareness of the dangers involved, no
fatalities attributable to hydrogen sulfide have occurred in Rotorua
since 1962 (Thom & Douglas, 1976).
Air pollution monitoring for hydrogen sulfide has been conducted,
during several periods over the past 15 years, at various sites in
Rotorua. However, there has been considerable variation in the results
obtained between the various sites, and furthermore, the
concentrations measured showed considerable seasonal variations. Some
of the results of these measurements are mentioned in section 4.1.
In 1964, the Division of Air Pollution, US Public Health Service,
reported that in the city of Terre Haute, Indiana, biodegradation of
industrial wastes in a 14.5-ha lagoon caused the atmospheric
concentration of hydrogen sulfide to reach a 1-h mean concentration of
0.45 mg/m3 (0.3 ppm). As a result, 81 complaints were registered by
the public, 41 of which were health-related. The most common
complaints were concerned with the perception of a foul odour. The
most common effects were nausea, interruption of sleep, burning of the
eyes, and shortness of breath. Less common manifestations were cough,
headache, and anorexia. Several acute asthma attacks were reported,
but the association of these attacks with the hydrogen sulfide
incident was not clearly established. Though no grave physical
illnesses could be directly related to the air pollution incident, the
investigators stressed that the odours emanating from the lagoon
caused more than a mere nuisance (United States Public Health Service,
1964).
The Poza Rica tragedy provides ample evidence that the accidental
release of hydrogen sulfide into a community can be expected to cause
systemic intoxication of varying severity. The Rotorua experience is
notable in that it emphasizes the fact that the potential for serious,
even fatal, hydrogen sulfide intoxication is present in active
geothermal areas. The more common picture of general population
exposure is exemplified by the Terre Haute incident where an industry-
related source of low-level concentrations of hydrogen sulfide gas
created a health problem which, although not grave, exceeded the level
of mere nuisance.
The potential of long-term, low-level exposure to hydrogen sulfide
to cause pulmonary changes of the type known to be associated with
other irritant gases such as oxides of nitrogen and sulfur has
scarcely been studied. No epidemiological data are available, at
present, upon which to base any sound conclusions.
7. EVALUATION OF HEALTH RISKS TO MAN FROM EXPOSURE TO HYDROGEN SULFIDE
By far the most important recognized toxic effect of hydrogen
sulfide is its ability to induce acute intoxication, characterized by
immediate collapse, frequently accompanied by respiratory arrest and,
without treatment, death. The scientific literature abounds with cases
of this type, most often associated with industrial overexposure.
However, a few cases of acute hydrogen sulfide exposure have been
recorded in the general population as a result of the release of
hydrogen sulfide either from an industrial process or from natural
sources. A second form of injury associated with exposure to hydrogen
sulfide is caused by the irritative action of the gas on the mucous
membranes of the eyes and respiratory tract. Keratoconjunctivitis (gas
eye) and pulmonary oedema are two most serious manifestations of this
local irritative effect. The malodorous property of hydrogen sulfide
gas is well recognized and this characteristic alone is believed by
many to be capable of producing impairment of human health and well-
being.
Most of the information available on human health effects
associated with exposure to various concentrations of hydrogen sulfide
gas has come from observations on accidental and industrial exposures.
With the exception of the Poza Rica catastrophe, information on
general population exposures and associated health effects is sketchy
at best. There is also little information on controlled human exposure
to hydrogen sulfide gas, and, except for data from odour threshold
studies, the information that is available is more than 50 years old.
Although a small amount of information is available on the effects on
experimental animals of high concentrations of hydrogen sulfide gas,
there is virtually no information on the long-term, low-level effects
of the gas on experimental animals. Furthermore, epidemiological data
are lacking concerning the health consequences of long-term, low-level
exposure to hydrogen sulfide, in both the general and industrial
populations.
7.1 Exposure Levels
General population air pollution problems associated with hydrogen
sulfide arise mainly in connexion with malodorous conditions,
traceable to point sources. Such sources can be industrial or, in some
cases, polluted bodies of walter. Peak levels as high as 0.20 mg/m3
(0.13 ppm) have been reported in the air in the neighbourhood of
industrial sources. Hydrogen sulfide is also a common pollutant in
geothermally active areas. At one site is a geothermal area in New
Zealand, where continuous measurements were carried out over a 5-month
period, a level of 0.08 mg/m3 (0.05 ppm) was exceeded, on average,
for 35% of the time.
Concentrations of hydrogen sulfide in the workplace also vary
widely. In the shale oil industry and in viscose rayon production, for
example, maximum levels of exposure during the work day have been
reported to range from 23 to 30 mg/m3 (15-20 ppm). In general,
however, massive accidental exposure to hydrogen sulfide has
constituted the principal hazard of this gas in industrial settings.
In many cases, such exposure has occurred because of equipment
breakage or malfunction. However, because hydrogen sulfide is heavier
than air, it can accumulate in lethal concentrations in low-lying or
enclosed areas. Numerous fatalities have occurred from the slow,
insidious accumulation of the gas in the air in both ambient and
industrial environments.
7.2 Experimental Animal Studies
The toxic effects of hydrogen sulfide gas have not been studied
extensively in experimental animals. However, in studies on a number
of animal species including the mouse, rat, cat, dog, and goat, it has
been shown that the primary target of hydrogen sulfide in high doses
is the nervous system. Collapse, followed by respiratory arrest and
asphyxia resulting from the paralysing effects of high concentrations
of hydrogen sulfide on the respiratory centres of the central nervous
system, is the usual sequence of events leading to death.
Little information is available in the published literature
concerning the effects in experimental animals of long-term, low-level
exposure to hydrogen sulfide.
7.3 Effects of Occupational Exposure
Inadvertent and accidental exposure of human subjects to high
concentrations of hydrogen sulfide has occurred among workers engaged
in petroleum refining, viscose rayon production, sugar beet
processing, and tannery work. Exposure levels have not been precisely
documented in many of these situations. Reported effects range from
the relatively less grave conditions of neurasthenic and
otoneurological symptoms and keratoconjunctivitis to the more serious
effects of pulmonary oedema, respiratory failure, collapse, and even
death. From the available data, it can be estimated that exposure for
seconds or minutes to concentrations of approximately 1400 mg/m3
(1000 ppm) or more would cause acute intoxication, concentrations of
140-1400 mg/m3 (100-1000 ppm) with an exposure time of up to several
hours would produce keratoconjunctivitis and pulmonary oedema, and
intermittent exposures to concentrations of 70-140 mg/m3
(50-100 ppm) could be associated with lingering, largely subjective,
manifestations believed by some to represent chronic intoxication.
Various studies have associated exposure to hydrogen sulfide in
concentrations as low as 16-32 mg/m3 (10.5-21.0 ppm) for several
hours with eye irritation in workers.
Effects of low-level, long-term industrial exposure to hydrogen
sulfide have not been systematically evaluated.
7.4. Effects of General Population Exposure
Several episodes of exposure of the general population to hydrogen
sulfide emanating from a specific source have been investigated and
described. For the most part, these events involved only annoyance
because of the odour or, at worst, minor temporary illness such as
headache, nausea, and sleeplessness. However, as described in section
6.3, on two occasions, general population exposure to hydrogen sulfide
caused grave illness and even death: one at Poza Rica, Mexico, and the
other in and around Rotorua, New Zealand.
Unfortunately, these incidents were not studied using
epidemiological techniques, and it is not possible to establish
exposure-effect relationships from the data.
7.5 Guidelines for the Protection of Public Health
There are two aspects concerning the protection of public health
in relation to hydrogen sulfide exposure: (a) the protection of the
public, and occupational groups in particular, from the toxicological
effects of such exposure; and (b) the protection of the public from
the odour nuisance that can be associated with releases of hydrogen
sulfide.
The odour threshold for hydrogen sulfide has been variously
reported to range from 0.0007 mg to 0.20 mg/m3 (0.0005-0.13 ppm)
(Table 2). Little information is available on the odour detection
limits for ambient hydrogen sulfide either under experimental field
conditions or in general population exposures. The Task Group
considered that a level of 0.007 mg/m3 (0.005 ppm) averaged over
30 min should not produce odour nuisance in most situations. Some
regulatory bodies may wish to adopt longer averaging times with
appropriately adjusted concentration limits.
The best estimates available suggest that eye irritation may occur
in man after several hours' exposure to hydrogen sulfide
concentrations of 16-32 mg/m3 (10.5-21.0 ppm). As occupational
exposure guidelines, the Task Group recommended the adoption of
10 mg/m3 (7 ppm) as a workshift time-weighted average value together
with a short-term exposure limit of 15 mg/m3 (10 ppm). The short-
term limit should be determined as a 10-min or less averaged value.
These limits should prevent eye irritation in workers which represents
the earliest recognized toxic response in man.
Annex tables 1 and 2 contain various national standards or
recommendations for ambient air quality and occupational exposure
limits for hydrogen sulfide. As can be seen, there is considerable
consensus regarding occupational exposure limits and the
recommendations of the Task Group are generally consistent with the
national values. There is less agreement regarding ambient air quality
standards, possibly because of different values placed on the nuisance
value of odours.
REFERENCES
ADAMS, D. F., YOUNG, F. A., & LUHR, R. A. (1968) Evaluation of an odor
perception threshold test facility. Tappi, 51: 62A-67A.
ADELSON, L. & SUNSHINE, I. (1966) Fatal hydrogen sulfide intoxication.
Report of three cases occurring in a sewer. Arch. Pathol.,
81: 375-380.
AHLBORG, G. (1951) Hydrogen sulfide poisoning in shale oil industry.
Am. Med. Assoc. ind. Hyg. occup. Med., 3: 247-266.
AIHA (1965) Hydrogen sulfide. Anal. Abstr., 12: 2207.
ALEXANDER, M. (1974) Microbial formation of environmental pollutants.
Adv. appl. Microbiol., 18: 37-40.
AMERICAN CONFERENCE OF GOVERNMENTAL INDUSTRIAL HYGIENISTS (1972)
Air sampling instruments for evaluation of atmospheric
contaminants, 4th ed., Cincinnati, ACGIH.
ANDERSSON, G., BROSSET, C., & GRENNFELT, P. (1974) The stability of
emitted odorous compounds in the atmosphere. In: Turk, A.,
Johnston, J. W. Jr, & Moulton, D. G., ed. Human response to
environmental odors, New York, Academic Press.
ANONYMOUS (1952) Four workers overcome by hydrogen sulfide when
digging in marshy land. Occup. Health, 12: 39.
BAMESBERGER, W. L. & ADAMS, D. F. (1969) Improvements in the
collection of hydrogen sulfide in cadmium hydroxide suspension.
Environ. Sci. Technol., 3: 258-281.
BAMESBERGER, W. L. & ADAMS, D. F. (1969) Field comparison of the
coulometric, colorimetric, and lead acetate tape analysis methods
for sulfur-containing gases. Tappi, 52: 1302-1306.
BEASLEY, R. W. R. (1963) The eye and hydrogen sulfide. Br. J. ind.
Med., 20: 32-34.
BECK, J. F., CORMIER, F. & DONINI, J. C. (1979) The combined toxicity
of ethanol and hydrogen sulfide. Toxicol. Lett., 3: 311-313.
BECKER, W. J. (1979) On H2S emission and immission measuring
techniques. Staub-Reinhalt. Luft, 39: 169-174.
BERUASHVILI, S. A. (1977) Skin irritant effect of water containing
hydrogen sulfide and results of population studies. In: Annual
Report of the Natadse Institute of Sanitary and Hygiene. Tbilisi,
Vol. XII, pp. 60-63.
BERUASHVILI, S. A. (1979) The long-term effects of the thermal water,
used for the hot water-supply. In: Proceedings of the 4
Conferences of the Young Scientists and Specialists on the
Problem "Environmental Health", Baku, pp. 3-5.
BLACK, M. S., HERBST, R. P., & HITCHCOCK, D. R. (1978) Solid absorbent
preconcentration and gas chromatographic analysis of sulfur gases.
Anal. Chem., 50: 848-851.
BROWN, K. E. (1969) Some toxicological problems in a rubber industry.
Med. J. Aust., 1: 534-538.
BURNETT, W. W., KING, E. G., GRACE, M., & HALL, W. F. (1977) Hydrogen
sulfide poisoning: review of 5 years' experience. Can. Med.
Assoc. J., 117: 1277-1280.
CALIFORNIA AIR RESOURCES BOARD (1970) Ambient air quality standards,
Sacramento, California, California Air Resources Board, pp. 69-75.
CAMPBELL, C. L., DAWES, R. K., DEOLALKAR, S., & MERRITT, M. C. (1958)
Effect of certain chemicals in water on the flavor of brewed
coffee. Food Res., 23: 575-579.
COOPER, R. C., JENKINS, D., & YOUNG, L. Y. (1976) Aquatic
microbiology laboratory manual, Texas, University of Texas.
DENMEAD, C. F. (1962) Air pollution by hydrogen sulfide from a shallow
polluted tidal inlet, Auckland, New Zealand. In: Clean Air
Conference, Sydney, Australia.
ELKINS, H. B. (1939) Toxic fumes. Ind. Med., 8: 426-432.
ESPACH, R. H. (1950) Sources of hydrogen sulfide in Wyoming. Ind.
Eng. Chem., 42: 2235-2237.
EVANS, C. L. (1967) The toxicity of hydrogen sulfide and other
sulfides. Quart. J exp. Physiol., 52: 231-248.
FEDERAL MINISTER FOR HOME AFFAIRS (1974) [Technical instructions for
prevention of air pollution, issued 28 August 1974]. pp. 175
(in German).
FUKUI, S., NAITO, S., KANEKO, M., & KANNO, S. (1967) [Hygienic
chemical studies on public nuisance by injurious gases. X.
Improvement of absorption mixture and examination of the methylene
blue method for determination of hydrogen sulfide.] Hyg. Chem.,
13: 16-21 (in Japanese).
GILARDI, E. F. & MANGANELLI, R. M. (1963) A laboratory study of a lead
acetate-tile method for the quantitative measurement of low
concentrations of hydrogen sulfide. Air Pollut. Control Assoc.,
13: 305-309.
GOLDMAN, F. H., COLEMAN, A. L., ELKINS, H. B., SCHRENK, H. H., &
SMUCKER, C. A. (1940) II. Report of sub-committee on chemical
methods in air analysis. Sampling and sampling devices.
Am. public Health Assoc. Year Book, pp. 92-98.
GOLDMAN, F. H., COLEMAN, A. A., ELKINS, H. B., & SCHRENK, H. H. (1943)
II. Report of sub-committee on chemical methods in air analysis.
Cadmium and hydrogen sulfide. Am. J. public Health, 33: 862-864.
GRANAT, L., RODHE, H., & HALLBERG, R. O. (1976) The global sulfur
cycle. In: Svensson, B. H. & Söderlund, R., ed. Nitrogen,
phosphorus and sulfur -- global cycles, SCOPE report 7. Ecol.
Bull. (Stockholm), 22: 89-134.
HAGGARD, H. W. (1925) The toxicology of hydrogen sulfide. J. ind.
Hyg., 7: 113-121.
HURWITZ, L. J. & TAYLOR, G.I. (1954) Poisoning by sewer gas with
unusual sequelae. Lancet, 1: 1110-1112.
ILO (1971) Tanning, leather finishing. In: Encyclopedia of
Occupational Health and Safety, 2nd ed., Geneva, International
Labour Office, pp. 1381-1382.
ILO (1971-72) Hydrogen sulfide. In: Encyclopedia of Occupational
Health and Safety, 2nd ed., Geneva, International Labour Office,
pp. 697-699.
INSTITUTE OF HYGIENE & EPIDEMIOLOGY (1978) [Air pollution by H2 S
at Boom, Belgium.] Brussels, pp. 1-17 (L.V.M. BO.77) (in Dutch).
ISO (1978) Proposal to the SC3 for the determination of hydrogen
sulfide in air, Geneva, International Organization for
Standardization, 8 pp. (ISO/TC 146/SC3/WG3 N 2E (revised),
1978-06-14).
INTERSOCIETY COMMITTEE (1977 a) Tentative method of analysis for
hydrogen sulfide content of the atmosphere. In: Katz, M., ed.
Methods of air sampling & analysis, 2nd ed., pp. 676-681.
INTERSOCIETY COMMITTEE (1977 b) Tentative method of gas
chromatographic analysis for sulfur-containing gases in the
atmosphere (automatic method with flame photometer detector).
In: Katz, M., ed. Methods of air sampling & analysis, 2nd ed.,
pp. 722-737.
JACOBS, M. B. (1965) Recommended standard method for continuing air
monitoring for hydrogen sulfide. Ultramicrodetermination of
sulfides in the air. J. Air Pollut. Control Assoc., 15: 314-315.
JOHNSON, B. A. (1972) The evaluation of gas detector tube systems:
hydrogen sulfide. Am. Ind. Hyg. Assoc. J., 33: 811-812.
JUNGE, C. E. (1963) Air chemistry and radioactivity, New York,
Academic press, pp. 68-69 (International Geophysics series
Vol. 4).
KAIPAINEN, W. J. (1954) Hydrogen sulfide intoxication. Rapidly
transient changes in the electrocardiogram suggestive of
myocardial infarction. Ann. Med. intern. Fenn., 43: 97-101.
KATZ, M. (1977) Sulfur and its inorganic derivatives in the Canadian
environment, Ottawa, Associate Committee on Scientific Criteria
for Environmental Quality, National Research Council of Canada,
pp. 21-68 (Publ. No. NRCC 15015).
KELLOGG, W. W., CADLE, R. D., ALLEN, E. R., LAZRUS, A. L., & MARTELL,
E. A. (1972) The sulfur cycle. Man's contribution as compared to
natural sources of sulfur compounds in the atmosphere and oceans.
Science, 175: 587-596.
KEMPER, F. D. (1966) A near-fatal case of hydrogen sulfide poisoning.
Can. Med. Assoc. J., 94: 1130-1131.
KLEINFELD, M., GIEL, C., & ROSSO, A. (1964) Acute hydrogen sulfide
intoxication; an unusual source of exposure. Ind. Med. Surg.,
33: 656-660.
KOSMIDER, S., ROGALA, E., & PACHOLEK, A. (1967) Electrocardiographic
and histochemical studies of the heart muscle in acute
experimental hydrogen sulfide poisoning. Arch. Immunol. ther.
Exp., 15: 731-740.
KRASOVITSKAYA, M. L., MALJAROVA, L. K. & ZAPOROZEC, T. S. (1965)
[Contamination of the air in the area of a refinery and
petrochemical plant.] Gig. i Sanit., 4: 103-105 (in Russian).
LARSON, C. P., REBERGER, C. C., & WICKS, M. J. (1964) The purple brain
death. Med. Sci. Law, 4: 200-202.
LAWRENCE BERKELEY LABORATORY (1976) Instrumentation for environmental
monitoring: air pollution. Ambient air monitor, Multi-component
monitoring system for air pollution and SO2 monitor, California,
University of California.
LEHMANN, K. B. (1892) [Experimental studies on the effect on the body
of gases and vapours of technical and hygienic importance, Part V,
hydrogen sulfide.] Arch. Hyg., 14: 135-189 (in German).
LEICHNITZ, K. (1977) Air analyses by means of long-term detector
tubes. Dräger Rev., 40: 9-17.
LEONARDOS, G., KENDALL, D., & BARNARD, N. (1969) Odor threshold
determination of 53 odorant chemicals. J. Air Pollut. Control
Assoc., 19: 91-95.
LEVAGGI, D. A., SIU, W., & FELDSTEIN, M. (1972) Continuous
determination of H2S in the PPB range by a specific colorimetric
method. In: Proceedings of the Technicians International
Congress, N.Y., pp. 65-67.
LINDVALL, T. (1970) On sensory evaluation of odorous air pollutant
intensities. Measurements of odor intensity in the laboratory and
in the field with special reference to effluents of sulfate pulp
factories. Nord. Hyg. Tidskr., 51 (suppl.): 36-39.
MACALUSO, P. (1969) Hydrogen sulfide. In: Mark, H. F., McKetta, J. J.,
& Othmer, D. F., ed. Encyclopedia of chemical technology, 2nd
ed., N.Y., John Wiley & Sons.
McANALLEY, B. H., LOWRY, W. T., OLIVER, R. D., & GARRIOTT, J. C.
(1979) Determination of inorganic sulfide and cyanide in blood
using specific ion electrodes: application to the investigation of
hydrogen sulfide and cyanide poisoning. J. anal. Toxicol.,
3: 111-114.
McCABE, L. C. & CLAYTON, G. D. (1952) Air pollution by hydrogen
sulfide in Poza Rica, Mexico. An evaluation of the incident of
Nov. 24, 1950. Am. Med. Assoc. Arch. ind. Hyg. occup. Med.,
6: 199-213.
McGRAW-HILL ENCYCLOPEDIA OF SCIENCE & TECHNOLOGY (1960) Heavy water,
New York, McGraw-Hill Book Co. Inc., pp. 382-383.
McKEE, J. E. & WOLF, H. W. (ed.) (1971) Water quality criteria, 2nd
ed., California, The Resources Agency of California, State Water
Resources Control Board, 584 pp. (Publ. No. 3-A).
MERCADO, S. G. (1975) Geothermo-electric project of Cerro-Prieto:
contamination and basic protection. In: Proceedings of the Second
UN Symposium on the Development and Use of Geothermal Resources,
San Francisco, CA, pp. 1385-1393.
MILBY, T. H. (1962) Hydrogen sulfide intoxication. Review of the
literature and report of unusual accident resulting in two cases
of nonfatal poisoning. J. occup. Med., 4: 431-437.
MINER, S. (1969) Preliminary air pollution survey of hydrogen
sulfide. A literature review, Raleigh, NC, National air
pollution control adm. (Publ. No. APTD 69-37).
MINSTER, J. T. (1963) Meteorology. Atmospheric hydrogen sulfide
concentrations during the London fog of December 1962. Nature
(Lond.), 199: 474-475.
MITCHELL, C. W. & DAVENPORT, S. J. (1924) Hydrogen sulfide literature.
Public health Rep., 39 (1): 1-13.
NATIONAL RESEARCH COUNCIL, USA (1979) Subcommittee on hydrogen
sulfide. Hydrogen sulfide, Baltimore, University Park Press.
NATUSCH, D. F. S., SEWELL, J. R., & TANNER, R. L. (1974) Determination
of hydrogen sulfide in air -- an assessment of impregnated paper
tape methods. Anal. Chem., 46 (3): 410-415.
NESSWETHA, W. (1969) [Eye lesions caused by sulfur compounds.]
Arbeitsmed. Sozialmed. Arbeitshyg., 4:288-290 (in German).
NEWILL, V. A. (1977) Air quality standards. In: Stern, A. D., ed. Air
pollution, Vol. V, Air quality management, 3rd ed., New York,
Academic Press.
NIOSH (1977) Occupational exposure to hydrogen sulfide, Cincinnati,
OH, National Institute for Occupational Safety and Health (DHEW
(NIOSH) publ. No. 77-158).
NYMAN, H. Th. (1954) Hydrogen sulfide eye inflammation -- treatment
with cortisone. Ind. Med. Surg., 23: 161-162.
PARÉ, J. P. (1966) A new tape reagent for the determination of
hydrogen sulfide in air. J. Air Pollut. Control Assoc.,
16: 325-327.
PEREGUD, Y. A., GERNET, Y. V., & BYKHOOSKAYA, M. S. (1971) Rapid
methods for the determination of noxious substances in the air,
Springfield, VA, US Dept. of Commerce, National Technical
Information Service, (AD 126795).
PETRUN, N. M. (1966) [Penetration of hydrogen sulfide through skin and
its influence on gaseous exchange and energy metabolism.] In:
Pharmacol. Toxicol., 2nd ed., Kiev, Publishing House "Health"
("Zdorovie"), pp. 247-250 (in Russian).
PODA, G. A. (1966) Hydrogen sulfide can be handled safely. Arch.
environ. Health, 12: 795-800.
ROBINSON, E. & ROBBINS, R. C. (1970) Gaseous sulfur pollutants from
urban and natural sources. J. Air Pollut. Control Assoc.,
20: 233-235.
ROLFE, K. A. (1980) The air-pollution aspects of geothermal power
stations. N.Z. energy J., 53: 51-58.
RYAZANOV, V. A. (1962) Sensory physiology as basis for air quality
standards. Arch. environ. Health, 5: 480-494.
SANDERSON, H. P., THOMAS, R., & KATZ, M. (1966) Limitations of the
lead acetate impregnated paper tape method for hydrogen sulfide.
J. Air Pollut. Control Assoc., 16: 328-330.
SAVOLAINEN, H., TENHUNEN, R., ELOVAARA, R., & TOSSAVAINEN, A. (1980)
Cumulative biochemical effects of repeated subclinical hydrogen
sulfide intoxication in mouse brain. Int. Arch. occup. environ.
Health, 46: 87-92.
SAYERS, R. R., SMITH, N. A. C., FIELDNER, A. C., MITCHELL, C. W.,
JONES, G. W., YANT, W. P., STARK, D. D., KATZ, S. H., BLOOMFIELD,
J. J., & JACOBS, W. A. (1925) Investigation of toxic gases from
Mexican and other high-sulfur petroleums and products. Report by
the Dept. of the Interior, Bureau of Mines, to the American
Petroleum Institute, Washington, DC, US Government Printing
Office, pp. 59-80 (Bull. No. 231).
SHAW, J. A. (1940) Rapid determination of hydrogen sulfide and
mercaptan sulfur. In gases and in aqueous solutions. Anal. Chem.,
12: 668-671.
SIMSON, R. E. & SIMPSON, G. R. (1971) Fatal hydrogen sulfide poisoning
associated with industrial waste exposure. Med. J. Aust.,
1: 331-334.
SIU, W., LEVAGGI, D. A., POTTER, L., MARTIN, R., & FELDSTEIN, M.
(1971) Modifications to an H2S tape sampler for increasing
sensitivity and accuracy in H2S sampling. J. Air Pollut.
Control Assoc., 21 (10): 636-638.
SMITH, R. P. & GOSSELIN, R. E. (1979) Hydrogen sulfide poisoning.
J. occup. Med., 21: 93-97.
STEVENS, R. K., MULIK, J. D., O'KEEFFE, A. E., & KROST, K. J. (1971)
Gas chromatography of reactive sulfur gases in air at the parts-
per-billion level. Anal. Chem., 43: 827-831.
STINE, R. J., SLOSBERG, B., & BEACHAM, B. E. (1976) Hydrogen sulfide
intoxication. A case report and discussion of treatment. Ann.
intern. Med., 85 (6): 756-758.
SUSMAN, J. L., HORNIG, J. F., THOMAE, S. C., & SMITH, R. P. (1978)
Pulmonary excretion of hydrogen sulfide, methanethiol, dimethyl
sulfide and dimethyl disulfide in mice. Drug Chem. Toxicol.,
1: 327-338.
THIELE, V. von (1979) [Experimental investigations to determine an
odour threshold value for hydrogen sulfide.] Staub-Reinhalt.
Luft, 39: 159-160 (in German).
THOM, N. G. & DOUGLAS, R. T. (1976) A study of hydrogen sulfide levels
in the geothermal areas of Rotorua, New Zealand. In: Fourth
International Clean Air Congress, Tokyo, pp. 565-568.
THOMPKINS, F. C., Jr & BECKER, J. H. (1976) An evaluation of
portable, direct-reading H2 S meters, Research Report,
Cincinnati, OH, National Institute for Occupational Safety and
Health (DHEW (NIOSH) Publ. No. 77-137).
UNITED STATES PUBLIC HEALTH SERVICE (1941) Hydrogen sulfide: its
toxicity and potential dangers, Cincinnati, OH, US Dept of
Health, Education, and Welfare, Public Health Service, Division of
Air Pollution, pp. 684-693.
UNITED STATES PUBLIC HEALTH SERVICE (1964) The air pollution
situation in Terre Haute, Indiana with special reference to the
hydrogen sulfide incident of May - June, 1964, Cincinnati, OH,
US Dept of Health, Education, and Welfare, Public Health Service
Division of Air Pollution, pp. 1-28.
WEAST, R. C., ed. (1977-78) Physical constants of inorganic compounds.
In: Handbook of chemistry and physics, 58th ed., Cleveland, OH,
USA, CRC Press.
WEST, P. W. (1970) Analytical methods for the study of air pollution.
Pure appl. Chem., 21: 437-447.
WINDHOLZ, M., ed. (1976) Hydrogen sulfide. In: Merck index, 9th ed.,
Rahway, N. J., Merck & Co. Inc., pp. 633-634.
WINNEKE, VON G., KOTALIK, J., KELDENICH, H.-O., & KASTKA, J. (1979)
[The identification of hydrogen sulfide under laboratory and field
conditions.] Staub-Reinhalt. Luft, 39:156-159 (in German).
YANT, W. P. (1930) Hydrogen sulfide in industry, occurrence, effects,
and treatment. Am. J. public Health, 20: 598-608.
Annex
Annex table 1. Ambient air quality standards for hydrogen sulfide from selected countries
Long-term Short-term
Country averaging averaging Reference
mg/m3 ppm time(h) mg/m3 ppm time(min)
Bulgaria 0.008 0.006 24 0.008 0.006 30 Newill (1977)
China -- -- -- 0.01 0.007 20 Official
Communicationd
Czechoslovakia 0.008 0.006 24 0.008 0.006 30 Newill (1977)
German 0.008 0.006 24 0.015 0.011 10--30 Newill (1977)
Democratic
Republic
Federal
Germany, 0.005 0.004 24 0.01 0.007 30 Minister for
Federal Home Affairs
Republic of (1974)
Hungary 0.008a 0.006 24 0.008a 0.008 30 Newill (1977)
Hungary 0.15 0.11 24 0.3 0.21 30 Newill (1977)
Israel 0.045 0.032 24 0.15 0.11 30 Newill (1977)
Philippines -- -- -- 0.3 0.21 30 UNEP/IRPTCe
Poland 0.008b 0.006 24 0.0086 0.008 30 UNEP/IRPTCe
Poland 0.02c 0.014 24 0.06c 0.04 20 UNEP/IRPTCe
Romania 0.01 0.007 24 0.03 0.02 30 Newill (1977)
Spain 0.004 0.003 24 0.01 0.007 30 Newill (1977)
USSR 0.008 0.006 24 0.008 0.006 30 Newill (1977)
Yugoslavia 0.008 0.006 24 0.008 0.006 30 Newill (1977)
a For highly protected and protected areas.
b For especially protected areas, sanatoria, health resorts, sanctuaries, and
national parks.
c For protected areas, towns, and villages.
d Official communication from the Institute of Health, Chinese Academy of Medical
Sciences.
e Private communication.
Note by the WHO Task Group
Ambient Air Quality Standards have been set according to different
criteria in different countries, and these criteria may include, but
are not necessarily limited to the assessment of health effects.
Moreover, the limits themselves may have different meanings such as
the maximum acceptable level or the permissible level over a 10 to
30-min averaging time, etc.
Annex table 2. Occupational exposure standards for hydrogen sulfide
from selected countriesa
Country mg/m3 ppm Standard type
Australia 15 10 8-h TWAb
Belgium 15 10 8-h TWAb
Bulgaria 10 ceiling
China 10 ceiling
Czechoslovakia -- average 10 shiftc TWAb
maximum 20 10-min STELd
Finland 15 10 8-h TWAb
German Democratic Republic -- average 15 8.75-h TWA
short-term 15 30-min STELd
Germany, Federal Republic of 15 10 8-h TWAb
Hungary 10 8-h TWAb
Italy 10 shiftc TWAb
Japan 15 10 shiftc TWAb
Netherlands 15 10 shiftc TWAb
Poland 10 8-h TWAb
Romania -- average 10 shiftTWAb
maximum 15 ceiling
Sweden 15 10 shifte TWAb
Switzerland 15 10 8 to 9-h TWAb
USSR 10 less than 30-min STELd
USA -- occupational standard 20 ceiling except for one
10-min peak less than
50 ppm
ACGIH 15 10 8-h TWAb
21 15 15-min STELd
Yugoslavia 10 7 ceiling
a Abstracted from: ILO (1977).
b TWA = time-weighted average value.
c = a time-weighted value averaged over the entire shift or workday.
d STEL = short-term exposure limit.
See Also:
Toxicological Abbreviations
Hydrogen sulfide (ICSC)

 hydrogen sulfide (Espach, 1950). Coals can contain sulfur levels of up
to 80 g/kg and, occasionally, conditions arise in which hydrogen
sulfide is formed within such deposits. Thus, special precautions must
be taken in some mining operations as well as in the drilling and
extraction of natural gas and crude oils with significant sulfur
content.
Hydrogen sulfide can also be released by activities surrounding
the development and use of geothermal resources. At the Cerro Prieto
geothermal power generating plant in Baja California, Mexico, for
example, hydrogen sulfide levels are sufficiently high to necessitate
special ventilation to protect electrical systems, and alarms for the
protection of personnel (Mercado, 1975).
During industrial operations, hydrogen sulfide can be formed
whenever elemental sulfur or certain sulfur-containing compounds come
into contact with organic materials at high temperatures. It is
usually produced as an undesirable by-product, though it is also used
as an important reagent or desirable intermediate compound in some
industrial processes such as the manufacture of sulfides, sodium
hydrosulfide, and various organic sulfur compounds. Examples of
processes in which hydrogen sulfide occurs as a by-product include the
production of coke from sulfur-containing coal, the production of
carbon disulfide, the manufacture of viscose rayon in the Kraft
process for producing wood pulp (Macaluso, 1969) and sulfur extraction
by the Frasch process.
In refining sulfur-containing crude oils, about 80%-90% of the
divalent sulfur compounds of hydrogen and carbon are converted to
hydrogen sulfide. Both the hydrogen sulfide produced and that
occurring in other industrial, geothermal, or natural gas streams can
be recovered by one of a number of processes that can be classified as
either absorption-desorption processes or processes involving
oxidation to oxides or to elemental sulfur. The bulk of hydrogen
sulfide recovered in industrial processes is used to produce elemental
sulfur or sulfuric acid (Macaluso, 1969).
Large quantities of hydrogen sulfide are used in the production of
heavy water, which is employed as a moderator in some nuclear power
reactors. The process is based on enrichment of the deuterium content
of water by hydrogen sulfide in a gas/liquid ion exchange system,
followed by separation of heavy water and water by fractional
distillation (McGraw-Hill Encyclopedia of Science and Technology,
1960).
In the tanning industry, hydrogen sulfide is produced in the
process by which hair or wool is removed from the hides. This
typically involves deliming by adding ammonium chloride or ammonium
sulfate followed by pickling with sulfuric acid, and takes place in
large rotating drums. The gases evolved, including hydrogen sulfide,
are released from the drums on opening the hatches either to add
chemicals or to unload the treated hides, and also from the waste
waters (ILO, 1971).
As in the natural environment, hydrogen sulfide can be generated
by bacterial action in industrial or community settings in malodorous
and sometimes dangerous amounts.
In some countries, such as India and Sri Lanka, hydrogen sulfide
is produced in the process by which coconut fibres are separated from
the husk. This procedure involves the decomposition of the husks in
shallow ponds. The hydrogen sulfide is produced as a result of
microbiological decay processes.
4. ENVIRONMENTAL LEVELS AND EXPOSURE
4.1 Concentrations in Outdoor Air
There are few published data on either natural background or urban
air levels of hydrogen sulfide. Robinson & Robbins (1970) estimated
the average ambient air level of hydrogen sulfide to be 0.0003 mg/m3
(0.0002 ppm). This estimate supports the data of Minster (1963) who,
when sampling over a 2´-year period in northwest London, reported that
air levels of hydrogen sulfide were generally below 0.00015 mg/m3
(0.0001 ppm), under clear, fresh conditions. Minster (1963) also
reported that average summer levels ranged from 0.00015 to 0.0007
mg/m3 (0.0001-0.0005 ppm) and average winter levels from 0.0007 to
0.0015 mg/m3 (0.0005-0.001 ppm). Data collected from this same
station during the London fog of December 1962 indicated a hydrogen
sulfide concentration of up to 0.046 mg/m3 (0.033 ppm) on 6 December
during heavy fog (each sampling time was 32 min).
Measurements summarized by the US National Air Pollution Control
Administration show concentrations ranging from below 0.001 mg/m3 to
0.006 mg/m3 (0.0007 to 0.0042 ppm) at various urban locations in the
USA in the period 1951-64 (Miner, 1969). However, as sensitive and
standardized methods of sampling and analysis for hydrogen sulfide
were lacking during this period, there is some doubt about the
reliability of these data. Furthermore, the averaging times for the
data are not available.
Much higher concentrations of hydrogen sulfide have been measured
near point sources. In California, peak concentrations as high as
0.20 mg/m3 (0.13 ppm) were measured near a pulp and papermill at the
time of its commissioning (California Air Resources Board, 1970).
After operating for several months, levels fell to 0.015 mg/m3
(0.010 ppm) or less. The averaging time was not reported. Near a brick
works in Boom, Belgium, air levels of hydrogen sulfide were monitored
over a 6-month period. During this time, the average 24-h
concentration of hydrogen sulfide was 0.005 mg/m3 (0.003 ppm) with
occasional daily averages in excess of 0.017 mg/m3 (0.011 ppm)
(Institute of Hygiene & Epidemiology, 1978).
A major accidental release of hydrogen sulfide occurred at Poza
Rica, Mexico, in 1950 (McCabe & Clayton, 1952). Although no data could
be collected on environmental levels during this episode, numerous
fatalities occurred indicating that exposure levels were most likely
in excess of 1500-3000 mg/m3 (1000-2000 ppm). Further details of
this episode are given in section 6.3.
In the geothermally active areas in and around the city of
Rotorua, New Zealand, airborne concentrations of hydrogen sulfide are
usually sufficient to cause noticeable odours (Thom & Douglas, 1976).
At one site, for one day, a 1-h mean concentration of up to
2.0 mg/m3 (1.4 ppm) was reported (Thom & Douglas, 1976). Continuous
measurements taken at another site over a period of 5 months showed
that a concentration of 0.08 mg/m3 (0.05 ppm) was exceeded, on
average, 35% of the time. It was also found that there were
considerable seasonal variations in the hydrogen sulfide levels,
reflecting the fluctuating steam-use patterns and also changes in the
dispersive nature of the atmosphere. During the mid-winter months of
the 1978 monitoring period, a concentration of hydrogen sulfide in air
of 0.08 mg/m3 (0.05 ppm) was exceeded more than 55% of the time,
whereas, during warmer months, this concentration was exceeded less
than 20% of the time (Rolfe, 1980).
Also in New Zealand, fine discharge of industrial and domestic
liquid wastes into an inlet near Auckland created conditions in which
hydrogen sulfide levels were sufficient to cause paint blackening and
complaints of offensive odours. Continuous air monitoring was
conducted for 21 months. These data indicated that 40-min average
hydrogen sulfide concentrations in air of from 0.8 to 1.4 mg/m3
(0.5-0.96 ppm) occurred at some time during the worst months of the
year at all the sites monitored (Denmead, 1962).
4.2 Concentrations in Work Places
Under normal operating conditions, concentrations of hydrogen
sulfide in the air in work places are believed to be less than
10-15 mg/m3 (7-10 ppm), the 8-h time weighted average that most
national authorities have set as their occupational exposure standard
(Annex).
It is well known, however, that hazardous exposures to hydrogen
sulfide can occur under accidental circumstances in industries in
which gas streams with a high hydrogen sulfide content exist.
Furthermore, as hydrogen sulfide is slightly heavier than air, it can
accumulate in toxic concentrations in low-lying areas, even when
generated or leaking at very low rates. However, in such cases, the
environmental levels of hydrogen sulfide have usually only been
measured after the accidents in question, or have been determined by
simulation or reenactment. Concentrations that have been reported
range from 150 mg/m3 (100 ppm), in which a worker lost consciousness
while sawing ebonite boards (Brown, 1969), to 18 000 mg/m3
(12 000 ppm) in a case in which a truck driver died while cleaning the
tank of a vehicle used to transport industrial waste (Simson &
Simpson, 1971). In an outdoor setting, 4 workmen lost consciousness
while digging a pit in marshy land in which hydrogen sulfide
concentrations in air of 442-810 mg/m3 (295-540 ppm) were measured 5
days later (Anonymous, 1952). Alexander (1974) reported hydrogen
sulfide concentrations as high as 0.037 mg/litre (24.8 ppm) in a
sewage stabilization pond and 10.0-13.2 mg/m3 (6.7-8.8 ppm) in the
air 15 m from this pond. In a report by Ahlborg (1951) on hydrogen
sulfide poisoning in the Swedish shale oil industry, concentrations of
hydrogen sulfide measured at various locations in the plant ranged
from 30 to 900 mg/m3 (20-600 ppm), though men seldom worked where
the high levels occurred.
More recently, the US National Institute for Occupational Safety
and Health reported that, in viscose rayon churn rooms, spinning
tanks, and drying and storage cells, workers were mainly exposed
during the working day to hydrogen sulfide concentrations of
23 mg/m3 (15 ppm) or less with occasional peaks of 150 mg/m3
(100 ppm) (NIOSH, 1977).
In the USA, it has been estimated that there are 125 000 employees
potentially exposed to hydrogen sulfide (NIOSH, 1977), Table 1 is a
list of occupations in which such exposure can occur ranging according
to occupation from rare exposure to low concentrations, to frequent
exposure to concentrations very near those associated with adverse
health effects.
Table 1. Examples of occupations with potential exposure to
hydrogen sulfidea
Animal fat and oil processors Lithographers
Animal manure removers Lithopone makers
Artificial-flavour makers Livestock farmers
Asphalt storage workers Manhole and trench workers
Barium carbonate makers Metallurgists
Barium salt makers Miners
Blast furnace workers Natural gas production and
Brewery workers processing workers
Bromide-brine workers Painters using polysulflde
Cable splicers caulking compounds
Caisson workers Papermakers
Carbon disulfide makers Petroleum production and
Cellophane makers refinery workers
Chemical laboratory workers, Phosphate purifiers
teachers, students Photo-engravers
Cistern cleaners Pipeline maintenance workers
Citrus root fumigators Pyrite burners
Coal gasification workers Rayon makers
Coke oven workers Refrigerant makers
Copper-ore sulfidizers Rubber and plastics processors
Depilatory makers Septic tank cleaners
Dyemakers Sewage treatment plant workers
Excavators Sewer workers
Felt makers Sheepdippers
Fermentation process workers Silk makers
Fertilizer makers Slaughterhouse workers
Fishing and fish-processing workers Smelting workers
Fur dressers Soapmakers
Geothermal-power drilling and Sugar beet and cane processors
production workers Sulfur spa workers
Gluemakers Sulfur products processors
Gold-ore workers Synthetic-fibre makers
Heavy-metal precipitators Tank gaugers
Heavy-water manufacturers Tannery workers
Hydrochloric acid purifiers Textile printers
Hydrogen sulfide production Thiophene makers
and sales workers Tunnel workers
Landfill workers Well diggers and cleaners
Lead ore sulfidizers Wool pullers
Lead removers
a From: NIOSH (1977).
5. EFFECTS ON EXPERIMENTAL ANIMALS
Very little information is available on the effects of low level
concentrations of hydrogen sulfide gas on experimental animals; most
published data have emphasized the effects of exposure to lethal or
near-lethal concentrations of the gas. According to Evans (1967) and
Smith & Gosselin (1979), the effects of high doses of hydrogen sulfide
and high doses of cyanide are very similar. Both inhibit the enzyme
cytochrome c oxidase [EC 1.9.3.1]. This was demonstrated in studies
using purified preparations of the enzyme (Smith & Gosselin, 1979).
When sodium sulfide at 0.1, 0.25, and 0.32 mmol/kg body weight was
administered intraperitoneally to mice, sulfide was not exhaled
(Susman et al., 1978) suggesting that it was inactivated primarily by
metabolism. In studies on the inhalation of hydrogen sulfide in rats,
cats, rabbits, and dogs, the nervous centres were first excited arid
then paralysed; pupils first contracted and then dilated; blood
pressure was first raised, then lowered; and respiration first
increased and then halted (Evans, 1967; Haggard, 1925). The findings
of Lehmann (1892) in the cat, dog, and rabbit, of Haggard (1925) in
the dog, and of Sayers et al. (1925) in the canary, rat, guineapig,
dog, and goat, are quite consistent: at 150-225 mg/m3 (100-150 ppm),
signs of local irritation of eyes and throat after many hours of
exposure; at 300-450 mg/m3 (200-300 ppm), eye and mucous membrane
irritation after inhalation for 1 h and slight general effects with
prolonged inhalation; at 750-1050 mg/m3 (500-700 ppm), local
irritation and slight systemic symptoms in less than 1 h and possible
death after several hours' exposure; at 1350 mg/m3 (900 ppm), grave
systemic effects within 30 min and death in less than 1 h; at
2250 mg/m3 (1500 ppm), collapse and death within 15-30 min; and at
2700 mg/m3 (1800 ppm), immediate collapse, respiratory paralysis,
and death.
When mice were exposed repeatedly (4 times for 2 h, at 4-day
intervals) to a hydrogen sulfide concentration in air of 150 mg/m3
(100 ppm), the critical inhibition of terminal cytochrome c oxidase
appeared to be cumulative. This effect was accompanied by a cumulative
decrease in cerebral RNA synthesis (Savolainen et al., 1980).
Experimental studies on rabbits indicated that either a single or
repeated exposure for 1.5 h per day (5 consecutive days) to a hydrogen
sulfide concentration in air of 105 mg/m3 (70 ppm) caused
electrocardiographic (ECG) changes (Kósmider et al., 1967).
Though some small differences in susceptibility to hydrogen
sulfide gas were exhibited among the species studied by Sayers et al.,
(1925), canaries being the most sensitive and goats the most
resistant, the interspecies differences were slight. It is agreed
among investigators that the effects of hydrogen sulfide gas on the
nervous system represent the most important aspect of its toxicity
(Haggard, 1925; Evans, 1967). Beck et al. (1979) showed that ethanol
in doses of 0.33-0.66 g/kg significantly shortened the time to loss of
consciousness in rats exposed to a hydrogen sulfide concentration in
air of 1200 mg/m3 (800 ppm) for 30 min. The induction of
methaemoglobinaemia by the injection of sodium nitrite had both
protective and antidotal effects against hydrogen sulfide poisoning in
mice, armadillos, rabbits, and dogs (Smith & Gosselin, 1979).
Water containing a hydrogen sulfide concentration as low as
0.86 mg/litre was toxic to trout after exposure for 24 h (McKee &
Wolf, 1971).
6. EFFECTS ON MAN
Adequate systematic studies of the relationship between hydrogen
sulfide exposure and health status in the general population have not
been carried out. Controlled exposure of human subjects to
concentrations of hydrogen sulfide gas exceeding about 75 mg/m3
(50 ppm) has been deemed to involve excessive risk because of the
possibility of injury to the lungs (Sayers et al., 1925; National
Research Council, USA, 1979). Furthermore, except for studies related
to odour threshold, controlled exposures of human subjects to very low
concentrations of the gas, for example, below 1.5 mg/m3 (1.0 ppm)
have not been reported. Thus, the information presented in this
section has mainly been derived from reports of accidental and
industrial exposures to hydrogen sulfide. A general discussion of the
toxicology of hydrogen sulfide has been included, because a basic
understanding of the subject is necessary for a discussion of the role
of the gas as an industrial and community hazard.
6.1 General Toxicological Considerations
The following observations have been derived from reports of
studies involving man. However, for clarification, some studies on
experimental animals have also been included. In general, both animals
and man respond in a very similar fashion to toxic concentrations of
hydrogen sulfide. It is both an irritant and an asphyxiant gas
(Table 2) that induces local inflammation of the membranes of the
human eye and respiratory tract (Yant, 1930). It has been shown that
eye irritation, the most commonly reported effect of hydrogen sulfide
exposure, can occur after several hours' exposure to concentrations of
16-32 mg/m3 (10.5-21.0 ppm) (Elkins, 1939; Nesswetha, 1969).
However, pulmonary tract irritation is, potentially, a more serious
reaction. When inhaled by dogs, hydrogen sulfide exerted an irritant
action through the entire respiratory tract, though the deeper
structures suffered the greatest damage (Haggard, 1925). Inflammation
of these deeper structures may result in pulmonary oedema.
Exposure to hydrogen sulfide gas did not induce important effects
on the human skin nor was any appreciable absorption through intact
skin observed (Yant, 1930). However, Petrun (1966) reported that when
the skin of rabbits was exposed to hydrogen sulfide at concentrations
of 1050 and 2100 mg/m3 (700 and 1400 ppm), trace amounts of hydrogen
sulfide were found in the exhaled air of the rabbits. No quantitative
information was given.
Hydrogen sulfide gas is rapidly absorbed through the lung. Like
hydrogen cyanide, it is a potent inhibitor of cytochrome c oxidase
that interferes with tissue use (Smith & Gosselin, 1979). As a result,
the oxidative metabolism may slow to the point where tissue metabolic
demands cannot be met. In the central nervous system, the result may
be paralysis of the respiratory centres. Respiratory arrest and death
from asphyxia would be the natural outcome.
In studies on dogs (Haggard, 1925), hydrogen sulfide at
concentrations of 1500-3000 mg/m3 (1000-2000 ppm) initially
stimulated excessively rapid breathing (hyperpnoea), because of a
depletion in the carbon dioxide content of the blood (hypocapnia).
This was followed by a period of respiratory inactivity (apnoea).
Spontaneous respiration may be reestablished, if carbon dioxide
depletion has not progressed beyond the point where prompt
reaccumulation can act as a stimulus to the reestablishment of
respiration. If spontaneous recovery does not occur and artificial
respiration is not applied rapidly, death from asphyxia is the
inevitable result (Haggard, 1925). At about 2250 mg/m3 (1500 ppm),
the sequence of events in the dog was the same, except that the
reaction was more pronounced; at 3000 mg/m3 (2000 ppm) there was
respiratory paralysis after a breath or two and, in Haggard's words,
"the victim falls to the ground as though struck down". When breathing
ceases, generalized convulsions frequently begin. There appears to be
no clear explanation of the cause of this picture of sudden collapse.
According to Haggard (1925), this form of respiratory failure is not
related to the carbon dioxide content of the blood but, rather, to the
directly paralysing effect of hydrogen sulfide on the respiratory
centre. Breathing is never reestablished spontaneously following this
hydrogen sulfide-induced respiratory paralysis. Haggard noted,
however, that because the heart continues to beat for several minutes
after respiration has ceased, death from asphyxia can be prevented if
artificial respiration is begun immediately and is continued until the
hydrogen sulfide concentration in the blood decreases. This decrease
is probably a consequence of metabolic processes, as shown in mice by
Susman et al. (1978) rather than, as once believed, the result of the
pulmonary excretion of the gas.
Smith & Gosselin (1979) have called attention to the confusion
that exists in the literature with regard to the effects of hydrogen
sulfide on haemoglobin. They emphasize that many studies have proved
that neither sulfhaemoglobin nor any other abnormal pigments are
present in sufficient concentrations in the blood of animals or human
subjects, fatally poisoned by hydrogen sulfide.
The characteristic "rotten egg" odour of hydrogen sulfide is an
important aspect of the toxicology of the gas. The threshold of
perception (odour) varies considerably depending on individual
sensitivity. Several authors have reported odour detection thresholds
ranging from 0.0007 mg/m3 to 0.20 mg/m3 (0.0005-0.13 ppm)
(Table 2). Thus, the odour of hydrogen sulfide gas can be a very
sensitive indicator of its presence in low concentrations. However, at
higher concentrations (> 225 mg/m3 (150 ppm)), hydrogen sulfide
exerts a paralysing effect on the olfactory apparatus (Milby, 1962),
thus neutralizing the value of its odour as a warning signal. Poda
(1966) reported that among 42 workers, who were rendered unconscious
from overexposure to hydrogen sulfide, majority did not smell the
characteristic odour of the gas but noted a sickeningly sweet odour,
very briefly, before losing consciousness.
Table 2. Effects of hydrogen sulfide exposure at various concentrations in air
Concentration
Effect Duration of
mg/m3 ppm exposure Reference
Man
Approximate threshold 0.0007-0.2 0.0005-0.13 A few seconds Yant (1930); Ryazanov
for odour to less (1962); Adams & Young
than 1 min (1968); Leonardos et al.
(1969); Lindvall (1970);
Thiele (1979); Winneke
et al. (1979)
Threshold of eye 16-32 10.5-21 6-7 h Elkins (1939)
irritation Nesswetha (1969)
Acute conjuctivitis 75-150 50-100 > 1 h Yant (1930)
(gas eye)
Loss of sense of smell 225-300 150-200 2-15 min Sayers et al. (1925)
Animalsa
Local irritation and 750-1050 500-700 < 1 h Haggard (1925)
slight systemic symptoms;
possible death
after several hours
Table 2. (Con't)
Concentration
Effect Duration of
mg/m3 ppm exposure Reference
Systemic symptoms; 1350 900 < 30 min Haggard (1925)
death in less than 1 h
Death 2250 1500 15-30 min Haggard (1925)
a These observations were made in experimental animals. However, there are no better quantitative
data available concerning man with respect to exposure to hydrogen sulfide at high concentrations.
Hydrogen sulfide intoxication in man has generally been
categorized according to 3 clinical forms, acute, subacute, and
chronic depending on the nature of the predominant clinical signs and
symptoms (National Research Council, USA, 1979). The term "acute
hydrogen sulfide intoxication" has been most often used to describe
systemic poisoning characterized by rapid onset and predominance of
signs and symptoms of nervous system involvement. The term "subacute
intoxication" has been applied to episodes of poisoning in which signs
and symptoms of eye and respiratory tract irritation were most
prominent. "Chronic hydrogen sulfide intoxication" has been applied by
some authors to describe a prolonged state of symptoms resulting from
a single or repeated exposure to concentrations of hydrogen sulfide
that do not produce clear-cut manifestations of either acute or
subacute illness.
In a document prepared by the National Research Council of the
National Academy of Sciences of the USA (National Research Council,
USA, 1979), the observation was made that application of the terms
"acute", "subacute" and "chronic" to hydrogen sulfide exposure was
both imprecise and misleading. However, rather than abandon these
frequently used terms altogether, the authors suggested a series of
clarifying definitions which are quoted in the following paragraphs,
and will henceforth be used in this section.
" Acute intoxication: Effects of a single exposure [seconds-
minutes]a to massive concentrations of hydrogen sulfide that rapidly
produce signs of respiratory distress. Concentrations approximating
1400 mg/m3 (1000 ppm) are usually required to cause acute
intoxication.
Sub-acute intoxication: Effects of continuous exposure [up to
several hours]a to mid-level 140 to 1400 mg/m3 (100 to 1000 ppm)
concentrations of hydrogen sulfide. Eye irritation (gas eye) is the
most commonly reported effect, but pulmonary edema (in the absence of
acute intoxication) has also been noted.
Chronic intoxication: Effects of intermittent exposures to low
to intermediate concentrations 70 to 140 mg/m3 (50 to 100 ppm) of
hydrogen sulfide, characterized by "lingering", largely subjective
manifestations of illness."
It is important to note that these definitions do not include a
consideration of the health consequences to man associated with
prolonged low-level exposure to hydrogen sulfide gas, such as may be
encountered under conditions of general urban air pollution.
A concentration of hydrogen sulfide in drinking water as low as
0.07 µg/litre (0.05 ppm) can affect its taste (Campbell et al., 1958).
a Time factors added by WHO Task Group.
6.2 Occupational Exposure
In certain occupations, workers are intermittently exposed to
concentrations of hydrogen sulfide that are not only malodorous but
can, in some situations, cause severe adverse health effects and even
death. Usually, hydrogen sulfide is encountered in the workplace as an
undesirable by-product of a manufacturing process, notably petroleum
refining, viscose rayon production, sugar beet processing, and tannery
work (Milby, 1962; NIOSH, 1977). In other occupations, for example
cesspool cleaning and work in sewers, exposure to hydrogen sulfide may
occur when the gas is formed as a result of the decomposition of
sulfur-containing organic matter, in the absence of complete
oxidation. Deaths attributed to such exposure occurred in the sewers
of Paris in the 1780s (Mitchell & Davenport, 1924) and still occur
under various circumstances.
Workers in certain occupations risk exposure to naturally
occurring hydrogen sulfide; geothermal energy workers and spa
attendants may be included in this category.
Acute hydrogen sulfide intoxication is a dramatic, often fatal
event. Three men were inadvertently enveloped in a cloud of hydrogen
sulfide gas escaping from a cylinder under high pressure; all fell, as
if struck down, and ceased breathing. Only as a result of prompt
resuscitation by trained onlookers did the men survive, though the two
most seriously affected experienced violent convulsions and did not
recover consciousness for some 30 min. None of the men suffered
important after-effects, and none recalled having noted the
characteristic odour of hydrogen sulfide. The hydrogen sulfide
concentrations to which the men were exposed were estimated to be
about 2800 mg/m3 (2000 ppm) (Milby, 1962). Twelve workmen in a plant
that produced benzyl polysulfide were overcome by hydrogen sulfide
gas, when a pipe used to transfer sodium sulfhydrate ruptured. The
liquid sulfhydrate drained into a nearby sewer, where it reacted with
acid sewage releasing hydrogen sulfide from several sewer openings in
the immediate vicinity. Two of the 12 workmen died, probably as a
result of respiratory arrest; 3 stopped breathing but were
successfully resuscitated; 6 lost consciousness but recovered
spontaneously, and 1 individual developed pulmonary oedema that
responded to therapy (Kleinfeld et al., 1964).
Burnett et al. (1977) reviewed 221 cases of exposure to hydrogen
sulfide associated with the oil, gas, and petrochemical industries in
Canada. The overall mortality was 6%; three-quarters of all victims
experienced a period of unconsciousness and 12% were comatose. A high
proportion of patients had other neurological signs and symptoms,
including altered behaviour patterns, confusion, vertigo, agitation,
or somnolence. Respiratory tract effects were second in frequency only
to neurological manifestations. Forty percent of all cases required
some form of respiratory assistance and 15% of all cases developed
pulmonary oedema. Less severely affected patients complained primarily
of headaches, sore eyes, or gastrointestinal upsets. There were no
recognizable sequelae among the survivors. Data on environmental
exposure levels were not reported.
Nearly fatal cases of acute hydrogen sulfide intoxication
associated with sequelae of varying severity have been reported. A
48-year-old farmer, who collapsed from hydrogen sulfide intoxication
while shovelling manure, continued to have convulsive seizures after
resuscitation. The ECG changes suggested myocardial infarction. The
patient recovered but a slight, persistent dizziness remained
(Kaipainen, 1954). A 46-year-old sewer worker, who was overcome by
hydrogen sulfide in a manhole for 30 min, was cyanotic, suffering
generalized spasms, and required artificial respiration. A week later,
he could move and speak only with great effort; a month afterwards, he
still exhibited neurological deficits. The ECG showed evidence of
small anterolateral infarct and a right bundle branch block. Three
months later, although ambulatory, the patient still suffered from
anginal pain upon exertion (Hurwitz & Taylor, 1954). In a man who had
suffered severe hydrogen sulfide intoxication with collapse and
respiratory failure, ECG evidence of myocardial ischaemia was noted
during the early phase of acute illness but gradually disappeared over
a period of 15 days (Kemper, 1966). Each of these 3 events, in which
sequelae were reported, were characterized by periods of
unconsciousness during which hypoxia of vital issues was likely to
have occurred and may have been the basis for the observed prolonged
effects. Many other instances of sequelae following acute hydrogen
sulfide intoxication have been reported. Most resemble the cases just
mentioned, in which serious poisoning with unconsciousness preceded
the appearance of sequelae.
Ahlborg (1951) described 58 cases of acute hydrogen sulfide
intoxication in Sweden's shale oil industry. There were no fatalities.
Symptoms were generally uniform: sudden fatigue, dizziness, and
intense anxiety followed by unconsciousness with or without
respiratory failure. Several cases of acute poisoning were not
associated with unconsciousness, otherwise symptoms were similar to
those of the other cases. The diagnosis of "sequelae after acute
hydrogen sulfide poisoning" was made in 15 cases. The majority of
these had a history of repeated acute intoxications followed in each
case by neurasthenic problems (fatigue, somnolence, headache, lack of
initiative, irritability, anxiety and poor memory, and decreased
libido), though some developed sequelae following acute intoxication
without an intercurrent episode of unconsciousness. Many stricken
workers developed an increase in sensitivity (aversion) to the odour
of gas of any type, even pure gasoline vapour (Ahlborg, 1951).
Numerous case histories of fatal hydrogen sulfide intoxication
have been reported (Larson et al., 1964; Adelson & Sunshine, 1966;
Simson & Simpson, 1971). Oedema of the lungs and brain are common
post-mortem findings. The presence of detectable concentrations of
hydrogen sulfide in the blood has been reported on several occasions
(Larson et al., 1964; Adelson & Sunshine, 1966) and concentrations in
the blood of fatally poisoned victims have ranged from 1.70 mg to
3.75 mg/litre (McAnalley et al., 1979).
In acute hydrogen sulfide intoxication, cessation of respiration
is an immediate threat to life. Accordingly, the provision of
artificially assisted respiration on an emergency basis is absolutely
critical. There is some question as to whether mouth-to-mouth
resuscitation may create a potential health hazard to the rescuer,
because of the presence of hydrogen sulfide in the expired air or on
the clothing of the victim. Thus, methods of artificial respiration
requiring less direct contact (for example, back-pressure-arm lift)
may be prudent.
6.3 General Population Exposure
There are several reports of episodes of general population
response to air contamination by hydrogen sulfide. Information derived
from these events is consistent with observations reported among
workers occupationally exposed to hydrogen sulfide. Table 2
demonstrates the wide range of odour perception thresholds for
hydrogen sulfide reported by various investigators. In view of the
magnitude of these differences, it is not possible to state with
certainty the concentration at which odour-related complaints can be
expected.
A catastrophic exposure episode involving the release of large
quantities of hydrogen sulfide into a small community was reported by
McCabe & Clayton (1952). This occurred in 1950 in Poza Rica, Mexico, a
city of 22 000 people located about 210 km northeast of Mexico City.
Poza Rica was then the centre of Mexico's leading oil-producing
district and the site of several oil field installations, including a
sulfur-recovery plant. An early morning malfunction of the waste gas
flare resulted in the release of large quantities of unburned hydrogen
sulfide into the atmosphere. The unburned gas, aided by a low-level
temperature inversion and light early morning breezes, was carried to
a residential area adjacent to the plant area. Residents of the area
were overcome while attempting to leave the area and while assisting
stricken neighbours. Within 3 h, 320 persons were hospitalized and 22
died. The most frequent symptom was loss of the sense of smell. More
than half of the patients lost consciousness, many suffered signs and
symptoms of respiratory tract and eye irritation and 9 developed
pulmonary oedema. Four of the 320 victims exhibited neurological
sequelae; 2 experienced neuritis of the acoustic nerve; 1 developed
dysarthria; the fourth patient suffered aggravation of pre-existing
epilepsy. The duration of these neurological sequelae was not
reported.
There have been reports of other episodes of general atmospheric
pollution by hydrogen sulfide evolved from both natural and industrial
sources, but none has been as severe as the Poza Rica incident.
In the geothermal areas of Rotorua, New Zealand, a city of 40 000
people, steam and hot water from approximately 500 active bores are
used to heat houses, buildings, and swimming pools, and to provide
domestic hot water supplies and steam for cooking boxes.
In these areas, accidental fatal cases of acute hydrogen sulfide
intoxication associated with improper ventilation of geothermal steam-
heated dwellings have occasionally been reported. For example, at
least 3 deaths from acute hydrogen sulfide poisoning occurred in 1962.
However, with the introduction of legal requirements regarding the
safe domestic use of the geothermal resources, and possibly also as a
result of increased public awareness of the dangers involved, no
fatalities attributable to hydrogen sulfide have occurred in Rotorua
since 1962 (Thom & Douglas, 1976).
Air pollution monitoring for hydrogen sulfide has been conducted,
during several periods over the past 15 years, at various sites in
Rotorua. However, there has been considerable variation in the results
obtained between the various sites, and furthermore, the
concentrations measured showed considerable seasonal variations. Some
of the results of these measurements are mentioned in section 4.1.
In 1964, the Division of Air Pollution, US Public Health Service,
reported that in the city of Terre Haute, Indiana, biodegradation of
industrial wastes in a 14.5-ha lagoon caused the atmospheric
concentration of hydrogen sulfide to reach a 1-h mean concentration of
0.45 mg/m3 (0.3 ppm). As a result, 81 complaints were registered by
the public, 41 of which were health-related. The most common
complaints were concerned with the perception of a foul odour. The
most common effects were nausea, interruption of sleep, burning of the
eyes, and shortness of breath. Less common manifestations were cough,
headache, and anorexia. Several acute asthma attacks were reported,
but the association of these attacks with the hydrogen sulfide
incident was not clearly established. Though no grave physical
illnesses could be directly related to the air pollution incident, the
investigators stressed that the odours emanating from the lagoon
caused more than a mere nuisance (United States Public Health Service,
1964).
The Poza Rica tragedy provides ample evidence that the accidental
release of hydrogen sulfide into a community can be expected to cause
systemic intoxication of varying severity. The Rotorua experience is
notable in that it emphasizes the fact that the potential for serious,
even fatal, hydrogen sulfide intoxication is present in active
geothermal areas. The more common picture of general population
exposure is exemplified by the Terre Haute incident where an industry-
related source of low-level concentrations of hydrogen sulfide gas
created a health problem which, although not grave, exceeded the level
of mere nuisance.
The potential of long-term, low-level exposure to hydrogen sulfide
to cause pulmonary changes of the type known to be associated with
other irritant gases such as oxides of nitrogen and sulfur has
scarcely been studied. No epidemiological data are available, at
present, upon which to base any sound conclusions.
7. EVALUATION OF HEALTH RISKS TO MAN FROM EXPOSURE TO HYDROGEN SULFIDE
By far the most important recognized toxic effect of hydrogen
sulfide is its ability to induce acute intoxication, characterized by
immediate collapse, frequently accompanied by respiratory arrest and,
without treatment, death. The scientific literature abounds with cases
of this type, most often associated with industrial overexposure.
However, a few cases of acute hydrogen sulfide exposure have been
recorded in the general population as a result of the release of
hydrogen sulfide either from an industrial process or from natural
sources. A second form of injury associated with exposure to hydrogen
sulfide is caused by the irritative action of the gas on the mucous
membranes of the eyes and respiratory tract. Keratoconjunctivitis (gas
eye) and pulmonary oedema are two most serious manifestations of this
local irritative effect. The malodorous property of hydrogen sulfide
gas is well recognized and this characteristic alone is believed by
many to be capable of producing impairment of human health and well-
being.
Most of the information available on human health effects
associated with exposure to various concentrations of hydrogen sulfide
gas has come from observations on accidental and industrial exposures.
With the exception of the Poza Rica catastrophe, information on
general population exposures and associated health effects is sketchy
at best. There is also little information on controlled human exposure
to hydrogen sulfide gas, and, except for data from odour threshold
studies, the information that is available is more than 50 years old.
Although a small amount of information is available on the effects on
experimental animals of high concentrations of hydrogen sulfide gas,
there is virtually no information on the long-term, low-level effects
of the gas on experimental animals. Furthermore, epidemiological data
are lacking concerning the health consequences of long-term, low-level
exposure to hydrogen sulfide, in both the general and industrial
populations.
7.1 Exposure Levels
General population air pollution problems associated with hydrogen
sulfide arise mainly in connexion with malodorous conditions,
traceable to point sources. Such sources can be industrial or, in some
cases, polluted bodies of walter. Peak levels as high as 0.20 mg/m3
(0.13 ppm) have been reported in the air in the neighbourhood of
industrial sources. Hydrogen sulfide is also a common pollutant in
geothermally active areas. At one site is a geothermal area in New
Zealand, where continuous measurements were carried out over a 5-month
period, a level of 0.08 mg/m3 (0.05 ppm) was exceeded, on average,
for 35% of the time.
Concentrations of hydrogen sulfide in the workplace also vary
widely. In the shale oil industry and in viscose rayon production, for
example, maximum levels of exposure during the work day have been
reported to range from 23 to 30 mg/m3 (15-20 ppm). In general,
however, massive accidental exposure to hydrogen sulfide has
constituted the principal hazard of this gas in industrial settings.
In many cases, such exposure has occurred because of equipment
breakage or malfunction. However, because hydrogen sulfide is heavier
than air, it can accumulate in lethal concentrations in low-lying or
enclosed areas. Numerous fatalities have occurred from the slow,
insidious accumulation of the gas in the air in both ambient and
industrial environments.
7.2 Experimental Animal Studies
The toxic effects of hydrogen sulfide gas have not been studied
extensively in experimental animals. However, in studies on a number
of animal species including the mouse, rat, cat, dog, and goat, it has
been shown that the primary target of hydrogen sulfide in high doses
is the nervous system. Collapse, followed by respiratory arrest and
asphyxia resulting from the paralysing effects of high concentrations
of hydrogen sulfide on the respiratory centres of the central nervous
system, is the usual sequence of events leading to death.
Little information is available in the published literature
concerning the effects in experimental animals of long-term, low-level
exposure to hydrogen sulfide.
7.3 Effects of Occupational Exposure
Inadvertent and accidental exposure of human subjects to high
concentrations of hydrogen sulfide has occurred among workers engaged
in petroleum refining, viscose rayon production, sugar beet
processing, and tannery work. Exposure levels have not been precisely
documented in many of these situations. Reported effects range from
the relatively less grave conditions of neurasthenic and
otoneurological symptoms and keratoconjunctivitis to the more serious
effects of pulmonary oedema, respiratory failure, collapse, and even
death. From the available data, it can be estimated that exposure for
seconds or minutes to concentrations of approximately 1400 mg/m3
(1000 ppm) or more would cause acute intoxication, concentrations of
140-1400 mg/m3 (100-1000 ppm) with an exposure time of up to several
hours would produce keratoconjunctivitis and pulmonary oedema, and
intermittent exposures to concentrations of 70-140 mg/m3
(50-100 ppm) could be associated with lingering, largely subjective,
manifestations believed by some to represent chronic intoxication.
Various studies have associated exposure to hydrogen sulfide in
concentrations as low as 16-32 mg/m3 (10.5-21.0 ppm) for several
hours with eye irritation in workers.
Effects of low-level, long-term industrial exposure to hydrogen
sulfide have not been systematically evaluated.
7.4. Effects of General Population Exposure
Several episodes of exposure of the general population to hydrogen
sulfide emanating from a specific source have been investigated and
described. For the most part, these events involved only annoyance
because of the odour or, at worst, minor temporary illness such as
headache, nausea, and sleeplessness. However, as described in section
6.3, on two occasions, general population exposure to hydrogen sulfide
caused grave illness and even death: one at Poza Rica, Mexico, and the
other in and around Rotorua, New Zealand.
Unfortunately, these incidents were not studied using
epidemiological techniques, and it is not possible to establish
exposure-effect relationships from the data.
7.5 Guidelines for the Protection of Public Health
There are two aspects concerning the protection of public health
in relation to hydrogen sulfide exposure: (a) the protection of the
public, and occupational groups in particular, from the toxicological
effects of such exposure; and (b) the protection of the public from
the odour nuisance that can be associated with releases of hydrogen
sulfide.
The odour threshold for hydrogen sulfide has been variously
reported to range from 0.0007 mg to 0.20 mg/m3 (0.0005-0.13 ppm)
(Table 2). Little information is available on the odour detection
limits for ambient hydrogen sulfide either under experimental field
conditions or in general population exposures. The Task Group
considered that a level of 0.007 mg/m3 (0.005 ppm) averaged over
30 min should not produce odour nuisance in most situations. Some
regulatory bodies may wish to adopt longer averaging times with
appropriately adjusted concentration limits.
The best estimates available suggest that eye irritation may occur
in man after several hours' exposure to hydrogen sulfide
concentrations of 16-32 mg/m3 (10.5-21.0 ppm). As occupational
exposure guidelines, the Task Group recommended the adoption of
10 mg/m3 (7 ppm) as a workshift time-weighted average value together
with a short-term exposure limit of 15 mg/m3 (10 ppm). The short-
term limit should be determined as a 10-min or less averaged value.
These limits should prevent eye irritation in workers which represents
the earliest recognized toxic response in man.
Annex tables 1 and 2 contain various national standards or
recommendations for ambient air quality and occupational exposure
limits for hydrogen sulfide. As can be seen, there is considerable
consensus regarding occupational exposure limits and the
recommendations of the Task Group are generally consistent with the
national values. There is less agreement regarding ambient air quality
standards, possibly because of different values placed on the nuisance
value of odours.
REFERENCES
ADAMS, D. F., YOUNG, F. A., & LUHR, R. A. (1968) Evaluation of an odor
perception threshold test facility. Tappi, 51: 62A-67A.
ADELSON, L. & SUNSHINE, I. (1966) Fatal hydrogen sulfide intoxication.
Report of three cases occurring in a sewer. Arch. Pathol.,
81: 375-380.
AHLBORG, G. (1951) Hydrogen sulfide poisoning in shale oil industry.
Am. Med. Assoc. ind. Hyg. occup. Med., 3: 247-266.
AIHA (1965) Hydrogen sulfide. Anal. Abstr., 12: 2207.
ALEXANDER, M. (1974) Microbial formation of environmental pollutants.
Adv. appl. Microbiol., 18: 37-40.
AMERICAN CONFERENCE OF GOVERNMENTAL INDUSTRIAL HYGIENISTS (1972)
Air sampling instruments for evaluation of atmospheric
contaminants, 4th ed., Cincinnati, ACGIH.
ANDERSSON, G., BROSSET, C., & GRENNFELT, P. (1974) The stability of
emitted odorous compounds in the atmosphere. In: Turk, A.,
Johnston, J. W. Jr, & Moulton, D. G., ed. Human response to
environmental odors, New York, Academic Press.
ANONYMOUS (1952) Four workers overcome by hydrogen sulfide when
digging in marshy land. Occup. Health, 12: 39.
BAMESBERGER, W. L. & ADAMS, D. F. (1969) Improvements in the
collection of hydrogen sulfide in cadmium hydroxide suspension.
Environ. Sci. Technol., 3: 258-281.
BAMESBERGER, W. L. & ADAMS, D. F. (1969) Field comparison of the
coulometric, colorimetric, and lead acetate tape analysis methods
for sulfur-containing gases. Tappi, 52: 1302-1306.
BEASLEY, R. W. R. (1963) The eye and hydrogen sulfide. Br. J. ind.
Med., 20: 32-34.
BECK, J. F., CORMIER, F. & DONINI, J. C. (1979) The combined toxicity
of ethanol and hydrogen sulfide. Toxicol. Lett., 3: 311-313.
BECKER, W. J. (1979) On H2S emission and immission measuring
techniques. Staub-Reinhalt. Luft, 39: 169-174.
BERUASHVILI, S. A. (1977) Skin irritant effect of water containing
hydrogen sulfide and results of population studies. In: Annual
Report of the Natadse Institute of Sanitary and Hygiene. Tbilisi,
Vol. XII, pp. 60-63.
BERUASHVILI, S. A. (1979) The long-term effects of the thermal water,
used for the hot water-supply. In: Proceedings of the 4
Conferences of the Young Scientists and Specialists on the
Problem "Environmental Health", Baku, pp. 3-5.
BLACK, M. S., HERBST, R. P., & HITCHCOCK, D. R. (1978) Solid absorbent
preconcentration and gas chromatographic analysis of sulfur gases.
Anal. Chem., 50: 848-851.
BROWN, K. E. (1969) Some toxicological problems in a rubber industry.
Med. J. Aust., 1: 534-538.
BURNETT, W. W., KING, E. G., GRACE, M., & HALL, W. F. (1977) Hydrogen
sulfide poisoning: review of 5 years' experience. Can. Med.
Assoc. J., 117: 1277-1280.
CALIFORNIA AIR RESOURCES BOARD (1970) Ambient air quality standards,
Sacramento, California, California Air Resources Board, pp. 69-75.
CAMPBELL, C. L., DAWES, R. K., DEOLALKAR, S., & MERRITT, M. C. (1958)
Effect of certain chemicals in water on the flavor of brewed
coffee. Food Res., 23: 575-579.
COOPER, R. C., JENKINS, D., & YOUNG, L. Y. (1976) Aquatic
microbiology laboratory manual, Texas, University of Texas.
DENMEAD, C. F. (1962) Air pollution by hydrogen sulfide from a shallow
polluted tidal inlet, Auckland, New Zealand. In: Clean Air
Conference, Sydney, Australia.
ELKINS, H. B. (1939) Toxic fumes. Ind. Med., 8: 426-432.
ESPACH, R. H. (1950) Sources of hydrogen sulfide in Wyoming. Ind.
Eng. Chem., 42: 2235-2237.
EVANS, C. L. (1967) The toxicity of hydrogen sulfide and other
sulfides. Quart. J exp. Physiol., 52: 231-248.
FEDERAL MINISTER FOR HOME AFFAIRS (1974) [Technical instructions for
prevention of air pollution, issued 28 August 1974]. pp. 175
(in German).
FUKUI, S., NAITO, S., KANEKO, M., & KANNO, S. (1967) [Hygienic
chemical studies on public nuisance by injurious gases. X.
Improvement of absorption mixture and examination of the methylene
blue method for determination of hydrogen sulfide.] Hyg. Chem.,
13: 16-21 (in Japanese).
GILARDI, E. F. & MANGANELLI, R. M. (1963) A laboratory study of a lead
acetate-tile method for the quantitative measurement of low
concentrations of hydrogen sulfide. Air Pollut. Control Assoc.,
13: 305-309.
GOLDMAN, F. H., COLEMAN, A. L., ELKINS, H. B., SCHRENK, H. H., &
SMUCKER, C. A. (1940) II. Report of sub-committee on chemical
methods in air analysis. Sampling and sampling devices.
Am. public Health Assoc. Year Book, pp. 92-98.
GOLDMAN, F. H., COLEMAN, A. A., ELKINS, H. B., & SCHRENK, H. H. (1943)
II. Report of sub-committee on chemical methods in air analysis.
Cadmium and hydrogen sulfide. Am. J. public Health, 33: 862-864.
GRANAT, L., RODHE, H., & HALLBERG, R. O. (1976) The global sulfur
cycle. In: Svensson, B. H. & Söderlund, R., ed. Nitrogen,
phosphorus and sulfur -- global cycles, SCOPE report 7. Ecol.
Bull. (Stockholm), 22: 89-134.
HAGGARD, H. W. (1925) The toxicology of hydrogen sulfide. J. ind.
Hyg., 7: 113-121.
HURWITZ, L. J. & TAYLOR, G.I. (1954) Poisoning by sewer gas with
unusual sequelae. Lancet, 1: 1110-1112.
ILO (1971) Tanning, leather finishing. In: Encyclopedia of
Occupational Health and Safety, 2nd ed., Geneva, International
Labour Office, pp. 1381-1382.
ILO (1971-72) Hydrogen sulfide. In: Encyclopedia of Occupational
Health and Safety, 2nd ed., Geneva, International Labour Office,
pp. 697-699.
INSTITUTE OF HYGIENE & EPIDEMIOLOGY (1978) [Air pollution by H2 S
at Boom, Belgium.] Brussels, pp. 1-17 (L.V.M. BO.77) (in Dutch).
ISO (1978) Proposal to the SC3 for the determination of hydrogen
sulfide in air, Geneva, International Organization for
Standardization, 8 pp. (ISO/TC 146/SC3/WG3 N 2E (revised),
1978-06-14).
INTERSOCIETY COMMITTEE (1977 a) Tentative method of analysis for
hydrogen sulfide content of the atmosphere. In: Katz, M., ed.
Methods of air sampling & analysis, 2nd ed., pp. 676-681.
INTERSOCIETY COMMITTEE (1977 b) Tentative method of gas
chromatographic analysis for sulfur-containing gases in the
atmosphere (automatic method with flame photometer detector).
In: Katz, M., ed. Methods of air sampling & analysis, 2nd ed.,
pp. 722-737.
JACOBS, M. B. (1965) Recommended standard method for continuing air
monitoring for hydrogen sulfide. Ultramicrodetermination of
sulfides in the air. J. Air Pollut. Control Assoc., 15: 314-315.
JOHNSON, B. A. (1972) The evaluation of gas detector tube systems:
hydrogen sulfide. Am. Ind. Hyg. Assoc. J., 33: 811-812.
JUNGE, C. E. (1963) Air chemistry and radioactivity, New York,
Academic press, pp. 68-69 (International Geophysics series
Vol. 4).
KAIPAINEN, W. J. (1954) Hydrogen sulfide intoxication. Rapidly
transient changes in the electrocardiogram suggestive of
myocardial infarction. Ann. Med. intern. Fenn., 43: 97-101.
KATZ, M. (1977) Sulfur and its inorganic derivatives in the Canadian
environment, Ottawa, Associate Committee on Scientific Criteria
for Environmental Quality, National Research Council of Canada,
pp. 21-68 (Publ. No. NRCC 15015).
KELLOGG, W. W., CADLE, R. D., ALLEN, E. R., LAZRUS, A. L., & MARTELL,
E. A. (1972) The sulfur cycle. Man's contribution as compared to
natural sources of sulfur compounds in the atmosphere and oceans.
Science, 175: 587-596.
KEMPER, F. D. (1966) A near-fatal case of hydrogen sulfide poisoning.
Can. Med. Assoc. J., 94: 1130-1131.
KLEINFELD, M., GIEL, C., & ROSSO, A. (1964) Acute hydrogen sulfide
intoxication; an unusual source of exposure. Ind. Med. Surg.,
33: 656-660.
KOSMIDER, S., ROGALA, E., & PACHOLEK, A. (1967) Electrocardiographic
and histochemical studies of the heart muscle in acute
experimental hydrogen sulfide poisoning. Arch. Immunol. ther.
Exp., 15: 731-740.
KRASOVITSKAYA, M. L., MALJAROVA, L. K. & ZAPOROZEC, T. S. (1965)
[Contamination of the air in the area of a refinery and
petrochemical plant.] Gig. i Sanit., 4: 103-105 (in Russian).
LARSON, C. P., REBERGER, C. C., & WICKS, M. J. (1964) The purple brain
death. Med. Sci. Law, 4: 200-202.
LAWRENCE BERKELEY LABORATORY (1976) Instrumentation for environmental
monitoring: air pollution. Ambient air monitor, Multi-component
monitoring system for air pollution and SO2 monitor, California,
University of California.
LEHMANN, K. B. (1892) [Experimental studies on the effect on the body
of gases and vapours of technical and hygienic importance, Part V,
hydrogen sulfide.] Arch. Hyg., 14: 135-189 (in German).
LEICHNITZ, K. (1977) Air analyses by means of long-term detector
tubes. Dräger Rev., 40: 9-17.
LEONARDOS, G., KENDALL, D., & BARNARD, N. (1969) Odor threshold
determination of 53 odorant chemicals. J. Air Pollut. Control
Assoc., 19: 91-95.
LEVAGGI, D. A., SIU, W., & FELDSTEIN, M. (1972) Continuous
determination of H2S in the PPB range by a specific colorimetric
method. In: Proceedings of the Technicians International
Congress, N.Y., pp. 65-67.
LINDVALL, T. (1970) On sensory evaluation of odorous air pollutant
intensities. Measurements of odor intensity in the laboratory and
in the field with special reference to effluents of sulfate pulp
factories. Nord. Hyg. Tidskr., 51 (suppl.): 36-39.
MACALUSO, P. (1969) Hydrogen sulfide. In: Mark, H. F., McKetta, J. J.,
& Othmer, D. F., ed. Encyclopedia of chemical technology, 2nd
ed., N.Y., John Wiley & Sons.
McANALLEY, B. H., LOWRY, W. T., OLIVER, R. D., & GARRIOTT, J. C.
(1979) Determination of inorganic sulfide and cyanide in blood
using specific ion electrodes: application to the investigation of
hydrogen sulfide and cyanide poisoning. J. anal. Toxicol.,
3: 111-114.
McCABE, L. C. & CLAYTON, G. D. (1952) Air pollution by hydrogen
sulfide in Poza Rica, Mexico. An evaluation of the incident of
Nov. 24, 1950. Am. Med. Assoc. Arch. ind. Hyg. occup. Med.,
6: 199-213.
McGRAW-HILL ENCYCLOPEDIA OF SCIENCE & TECHNOLOGY (1960) Heavy water,
New York, McGraw-Hill Book Co. Inc., pp. 382-383.
McKEE, J. E. & WOLF, H. W. (ed.) (1971) Water quality criteria, 2nd
ed., California, The Resources Agency of California, State Water
Resources Control Board, 584 pp. (Publ. No. 3-A).
MERCADO, S. G. (1975) Geothermo-electric project of Cerro-Prieto:
contamination and basic protection. In: Proceedings of the Second
UN Symposium on the Development and Use of Geothermal Resources,
San Francisco, CA, pp. 1385-1393.
MILBY, T. H. (1962) Hydrogen sulfide intoxication. Review of the
literature and report of unusual accident resulting in two cases
of nonfatal poisoning. J. occup. Med., 4: 431-437.
MINER, S. (1969) Preliminary air pollution survey of hydrogen
sulfide. A literature review, Raleigh, NC, National air
pollution control adm. (Publ. No. APTD 69-37).
MINSTER, J. T. (1963) Meteorology. Atmospheric hydrogen sulfide
concentrations during the London fog of December 1962. Nature
(Lond.), 199: 474-475.
MITCHELL, C. W. & DAVENPORT, S. J. (1924) Hydrogen sulfide literature.
Public health Rep., 39 (1): 1-13.
NATIONAL RESEARCH COUNCIL, USA (1979) Subcommittee on hydrogen
sulfide. Hydrogen sulfide, Baltimore, University Park Press.
NATUSCH, D. F. S., SEWELL, J. R., & TANNER, R. L. (1974) Determination
of hydrogen sulfide in air -- an assessment of impregnated paper
tape methods. Anal. Chem., 46 (3): 410-415.
NESSWETHA, W. (1969) [Eye lesions caused by sulfur compounds.]
Arbeitsmed. Sozialmed. Arbeitshyg., 4:288-290 (in German).
NEWILL, V. A. (1977) Air quality standards. In: Stern, A. D., ed. Air
pollution, Vol. V, Air quality management, 3rd ed., New York,
Academic Press.
NIOSH (1977) Occupational exposure to hydrogen sulfide, Cincinnati,
OH, National Institute for Occupational Safety and Health (DHEW
(NIOSH) publ. No. 77-158).
NYMAN, H. Th. (1954) Hydrogen sulfide eye inflammation -- treatment
with cortisone. Ind. Med. Surg., 23: 161-162.
PARÉ, J. P. (1966) A new tape reagent for the determination of
hydrogen sulfide in air. J. Air Pollut. Control Assoc.,
16: 325-327.
PEREGUD, Y. A., GERNET, Y. V., & BYKHOOSKAYA, M. S. (1971) Rapid
methods for the determination of noxious substances in the air,
Springfield, VA, US Dept. of Commerce, National Technical
Information Service, (AD 126795).
PETRUN, N. M. (1966) [Penetration of hydrogen sulfide through skin and
its influence on gaseous exchange and energy metabolism.] In:
Pharmacol. Toxicol., 2nd ed., Kiev, Publishing House "Health"
("Zdorovie"), pp. 247-250 (in Russian).
PODA, G. A. (1966) Hydrogen sulfide can be handled safely. Arch.
environ. Health, 12: 795-800.
ROBINSON, E. & ROBBINS, R. C. (1970) Gaseous sulfur pollutants from
urban and natural sources. J. Air Pollut. Control Assoc.,
20: 233-235.
ROLFE, K. A. (1980) The air-pollution aspects of geothermal power
stations. N.Z. energy J., 53: 51-58.
RYAZANOV, V. A. (1962) Sensory physiology as basis for air quality
standards. Arch. environ. Health, 5: 480-494.
SANDERSON, H. P., THOMAS, R., & KATZ, M. (1966) Limitations of the
lead acetate impregnated paper tape method for hydrogen sulfide.
J. Air Pollut. Control Assoc., 16: 328-330.
SAVOLAINEN, H., TENHUNEN, R., ELOVAARA, R., & TOSSAVAINEN, A. (1980)
Cumulative biochemical effects of repeated subclinical hydrogen
sulfide intoxication in mouse brain. Int. Arch. occup. environ.
Health, 46: 87-92.
SAYERS, R. R., SMITH, N. A. C., FIELDNER, A. C., MITCHELL, C. W.,
JONES, G. W., YANT, W. P., STARK, D. D., KATZ, S. H., BLOOMFIELD,
J. J., & JACOBS, W. A. (1925) Investigation of toxic gases from
Mexican and other high-sulfur petroleums and products. Report by
the Dept. of the Interior, Bureau of Mines, to the American
Petroleum Institute, Washington, DC, US Government Printing
Office, pp. 59-80 (Bull. No. 231).
SHAW, J. A. (1940) Rapid determination of hydrogen sulfide and
mercaptan sulfur. In gases and in aqueous solutions. Anal. Chem.,
12: 668-671.
SIMSON, R. E. & SIMPSON, G. R. (1971) Fatal hydrogen sulfide poisoning
associated with industrial waste exposure. Med. J. Aust.,
1: 331-334.
SIU, W., LEVAGGI, D. A., POTTER, L., MARTIN, R., & FELDSTEIN, M.
(1971) Modifications to an H2S tape sampler for increasing
sensitivity and accuracy in H2S sampling. J. Air Pollut.
Control Assoc., 21 (10): 636-638.
SMITH, R. P. & GOSSELIN, R. E. (1979) Hydrogen sulfide poisoning.
J. occup. Med., 21: 93-97.
STEVENS, R. K., MULIK, J. D., O'KEEFFE, A. E., & KROST, K. J. (1971)
Gas chromatography of reactive sulfur gases in air at the parts-
per-billion level. Anal. Chem., 43: 827-831.
STINE, R. J., SLOSBERG, B., & BEACHAM, B. E. (1976) Hydrogen sulfide
intoxication. A case report and discussion of treatment. Ann.
intern. Med., 85 (6): 756-758.
SUSMAN, J. L., HORNIG, J. F., THOMAE, S. C., & SMITH, R. P. (1978)
Pulmonary excretion of hydrogen sulfide, methanethiol, dimethyl
sulfide and dimethyl disulfide in mice. Drug Chem. Toxicol.,
1: 327-338.
THIELE, V. von (1979) [Experimental investigations to determine an
odour threshold value for hydrogen sulfide.] Staub-Reinhalt.
Luft, 39: 159-160 (in German).
THOM, N. G. & DOUGLAS, R. T. (1976) A study of hydrogen sulfide levels
in the geothermal areas of Rotorua, New Zealand. In: Fourth
International Clean Air Congress, Tokyo, pp. 565-568.
THOMPKINS, F. C., Jr & BECKER, J. H. (1976) An evaluation of
portable, direct-reading H2 S meters, Research Report,
Cincinnati, OH, National Institute for Occupational Safety and
Health (DHEW (NIOSH) Publ. No. 77-137).
UNITED STATES PUBLIC HEALTH SERVICE (1941) Hydrogen sulfide: its
toxicity and potential dangers, Cincinnati, OH, US Dept of
Health, Education, and Welfare, Public Health Service, Division of
Air Pollution, pp. 684-693.
UNITED STATES PUBLIC HEALTH SERVICE (1964) The air pollution
situation in Terre Haute, Indiana with special reference to the
hydrogen sulfide incident of May - June, 1964, Cincinnati, OH,
US Dept of Health, Education, and Welfare, Public Health Service
Division of Air Pollution, pp. 1-28.
WEAST, R. C., ed. (1977-78) Physical constants of inorganic compounds.
In: Handbook of chemistry and physics, 58th ed., Cleveland, OH,
USA, CRC Press.
WEST, P. W. (1970) Analytical methods for the study of air pollution.
Pure appl. Chem., 21: 437-447.
WINDHOLZ, M., ed. (1976) Hydrogen sulfide. In: Merck index, 9th ed.,
Rahway, N. J., Merck & Co. Inc., pp. 633-634.
WINNEKE, VON G., KOTALIK, J., KELDENICH, H.-O., & KASTKA, J. (1979)
[The identification of hydrogen sulfide under laboratory and field
conditions.] Staub-Reinhalt. Luft, 39:156-159 (in German).
YANT, W. P. (1930) Hydrogen sulfide in industry, occurrence, effects,
and treatment. Am. J. public Health, 20: 598-608.
Annex
Annex table 1. Ambient air quality standards for hydrogen sulfide from selected countries
Long-term Short-term
Country averaging averaging Reference
mg/m3 ppm time(h) mg/m3 ppm time(min)
Bulgaria 0.008 0.006 24 0.008 0.006 30 Newill (1977)
China -- -- -- 0.01 0.007 20 Official
Communicationd
Czechoslovakia 0.008 0.006 24 0.008 0.006 30 Newill (1977)
German 0.008 0.006 24 0.015 0.011 10--30 Newill (1977)
Democratic
Republic
Federal
Germany, 0.005 0.004 24 0.01 0.007 30 Minister for
Federal Home Affairs
Republic of (1974)
Hungary 0.008a 0.006 24 0.008a 0.008 30 Newill (1977)
Hungary 0.15 0.11 24 0.3 0.21 30 Newill (1977)
Israel 0.045 0.032 24 0.15 0.11 30 Newill (1977)
Philippines -- -- -- 0.3 0.21 30 UNEP/IRPTCe
Poland 0.008b 0.006 24 0.0086 0.008 30 UNEP/IRPTCe
Poland 0.02c 0.014 24 0.06c 0.04 20 UNEP/IRPTCe
Romania 0.01 0.007 24 0.03 0.02 30 Newill (1977)
Spain 0.004 0.003 24 0.01 0.007 30 Newill (1977)
USSR 0.008 0.006 24 0.008 0.006 30 Newill (1977)
Yugoslavia 0.008 0.006 24 0.008 0.006 30 Newill (1977)
a For highly protected and protected areas.
b For especially protected areas, sanatoria, health resorts, sanctuaries, and
national parks.
c For protected areas, towns, and villages.
d Official communication from the Institute of Health, Chinese Academy of Medical
Sciences.
e Private communication.
Note by the WHO Task Group
Ambient Air Quality Standards have been set according to different
criteria in different countries, and these criteria may include, but
are not necessarily limited to the assessment of health effects.
Moreover, the limits themselves may have different meanings such as
the maximum acceptable level or the permissible level over a 10 to
30-min averaging time, etc.
Annex table 2. Occupational exposure standards for hydrogen sulfide
from selected countriesa
Country mg/m3 ppm Standard type
Australia 15 10 8-h TWAb
Belgium 15 10 8-h TWAb
Bulgaria 10 ceiling
China 10 ceiling
Czechoslovakia -- average 10 shiftc TWAb
maximum 20 10-min STELd
Finland 15 10 8-h TWAb
German Democratic Republic -- average 15 8.75-h TWA
short-term 15 30-min STELd
Germany, Federal Republic of 15 10 8-h TWAb
Hungary 10 8-h TWAb
Italy 10 shiftc TWAb
Japan 15 10 shiftc TWAb
Netherlands 15 10 shiftc TWAb
Poland 10 8-h TWAb
Romania -- average 10 shiftTWAb
maximum 15 ceiling
Sweden 15 10 shifte TWAb
Switzerland 15 10 8 to 9-h TWAb
USSR 10 less than 30-min STELd
USA -- occupational standard 20 ceiling except for one
10-min peak less than
50 ppm
ACGIH 15 10 8-h TWAb
21 15 15-min STELd
Yugoslavia 10 7 ceiling
a Abstracted from: ILO (1977).
b TWA = time-weighted average value.
c = a time-weighted value averaged over the entire shift or workday.
d STEL = short-term exposure limit.
hydrogen sulfide (Espach, 1950). Coals can contain sulfur levels of up
to 80 g/kg and, occasionally, conditions arise in which hydrogen
sulfide is formed within such deposits. Thus, special precautions must
be taken in some mining operations as well as in the drilling and
extraction of natural gas and crude oils with significant sulfur
content.
Hydrogen sulfide can also be released by activities surrounding
the development and use of geothermal resources. At the Cerro Prieto
geothermal power generating plant in Baja California, Mexico, for
example, hydrogen sulfide levels are sufficiently high to necessitate
special ventilation to protect electrical systems, and alarms for the
protection of personnel (Mercado, 1975).
During industrial operations, hydrogen sulfide can be formed
whenever elemental sulfur or certain sulfur-containing compounds come
into contact with organic materials at high temperatures. It is
usually produced as an undesirable by-product, though it is also used
as an important reagent or desirable intermediate compound in some
industrial processes such as the manufacture of sulfides, sodium
hydrosulfide, and various organic sulfur compounds. Examples of
processes in which hydrogen sulfide occurs as a by-product include the
production of coke from sulfur-containing coal, the production of
carbon disulfide, the manufacture of viscose rayon in the Kraft
process for producing wood pulp (Macaluso, 1969) and sulfur extraction
by the Frasch process.
In refining sulfur-containing crude oils, about 80%-90% of the
divalent sulfur compounds of hydrogen and carbon are converted to
hydrogen sulfide. Both the hydrogen sulfide produced and that
occurring in other industrial, geothermal, or natural gas streams can
be recovered by one of a number of processes that can be classified as
either absorption-desorption processes or processes involving
oxidation to oxides or to elemental sulfur. The bulk of hydrogen
sulfide recovered in industrial processes is used to produce elemental
sulfur or sulfuric acid (Macaluso, 1969).
Large quantities of hydrogen sulfide are used in the production of
heavy water, which is employed as a moderator in some nuclear power
reactors. The process is based on enrichment of the deuterium content
of water by hydrogen sulfide in a gas/liquid ion exchange system,
followed by separation of heavy water and water by fractional
distillation (McGraw-Hill Encyclopedia of Science and Technology,
1960).
In the tanning industry, hydrogen sulfide is produced in the
process by which hair or wool is removed from the hides. This
typically involves deliming by adding ammonium chloride or ammonium
sulfate followed by pickling with sulfuric acid, and takes place in
large rotating drums. The gases evolved, including hydrogen sulfide,
are released from the drums on opening the hatches either to add
chemicals or to unload the treated hides, and also from the waste
waters (ILO, 1971).
As in the natural environment, hydrogen sulfide can be generated
by bacterial action in industrial or community settings in malodorous
and sometimes dangerous amounts.
In some countries, such as India and Sri Lanka, hydrogen sulfide
is produced in the process by which coconut fibres are separated from
the husk. This procedure involves the decomposition of the husks in
shallow ponds. The hydrogen sulfide is produced as a result of
microbiological decay processes.
4. ENVIRONMENTAL LEVELS AND EXPOSURE
4.1 Concentrations in Outdoor Air
There are few published data on either natural background or urban
air levels of hydrogen sulfide. Robinson & Robbins (1970) estimated
the average ambient air level of hydrogen sulfide to be 0.0003 mg/m3
(0.0002 ppm). This estimate supports the data of Minster (1963) who,
when sampling over a 2´-year period in northwest London, reported that
air levels of hydrogen sulfide were generally below 0.00015 mg/m3
(0.0001 ppm), under clear, fresh conditions. Minster (1963) also
reported that average summer levels ranged from 0.00015 to 0.0007
mg/m3 (0.0001-0.0005 ppm) and average winter levels from 0.0007 to
0.0015 mg/m3 (0.0005-0.001 ppm). Data collected from this same
station during the London fog of December 1962 indicated a hydrogen
sulfide concentration of up to 0.046 mg/m3 (0.033 ppm) on 6 December
during heavy fog (each sampling time was 32 min).
Measurements summarized by the US National Air Pollution Control
Administration show concentrations ranging from below 0.001 mg/m3 to
0.006 mg/m3 (0.0007 to 0.0042 ppm) at various urban locations in the
USA in the period 1951-64 (Miner, 1969). However, as sensitive and
standardized methods of sampling and analysis for hydrogen sulfide
were lacking during this period, there is some doubt about the
reliability of these data. Furthermore, the averaging times for the
data are not available.
Much higher concentrations of hydrogen sulfide have been measured
near point sources. In California, peak concentrations as high as
0.20 mg/m3 (0.13 ppm) were measured near a pulp and papermill at the
time of its commissioning (California Air Resources Board, 1970).
After operating for several months, levels fell to 0.015 mg/m3
(0.010 ppm) or less. The averaging time was not reported. Near a brick
works in Boom, Belgium, air levels of hydrogen sulfide were monitored
over a 6-month period. During this time, the average 24-h
concentration of hydrogen sulfide was 0.005 mg/m3 (0.003 ppm) with
occasional daily averages in excess of 0.017 mg/m3 (0.011 ppm)
(Institute of Hygiene & Epidemiology, 1978).
A major accidental release of hydrogen sulfide occurred at Poza
Rica, Mexico, in 1950 (McCabe & Clayton, 1952). Although no data could
be collected on environmental levels during this episode, numerous
fatalities occurred indicating that exposure levels were most likely
in excess of 1500-3000 mg/m3 (1000-2000 ppm). Further details of
this episode are given in section 6.3.
In the geothermally active areas in and around the city of
Rotorua, New Zealand, airborne concentrations of hydrogen sulfide are
usually sufficient to cause noticeable odours (Thom & Douglas, 1976).
At one site, for one day, a 1-h mean concentration of up to
2.0 mg/m3 (1.4 ppm) was reported (Thom & Douglas, 1976). Continuous
measurements taken at another site over a period of 5 months showed
that a concentration of 0.08 mg/m3 (0.05 ppm) was exceeded, on
average, 35% of the time. It was also found that there were
considerable seasonal variations in the hydrogen sulfide levels,
reflecting the fluctuating steam-use patterns and also changes in the
dispersive nature of the atmosphere. During the mid-winter months of
the 1978 monitoring period, a concentration of hydrogen sulfide in air
of 0.08 mg/m3 (0.05 ppm) was exceeded more than 55% of the time,
whereas, during warmer months, this concentration was exceeded less
than 20% of the time (Rolfe, 1980).
Also in New Zealand, fine discharge of industrial and domestic
liquid wastes into an inlet near Auckland created conditions in which
hydrogen sulfide levels were sufficient to cause paint blackening and
complaints of offensive odours. Continuous air monitoring was
conducted for 21 months. These data indicated that 40-min average
hydrogen sulfide concentrations in air of from 0.8 to 1.4 mg/m3
(0.5-0.96 ppm) occurred at some time during the worst months of the
year at all the sites monitored (Denmead, 1962).
4.2 Concentrations in Work Places
Under normal operating conditions, concentrations of hydrogen
sulfide in the air in work places are believed to be less than
10-15 mg/m3 (7-10 ppm), the 8-h time weighted average that most
national authorities have set as their occupational exposure standard
(Annex).
It is well known, however, that hazardous exposures to hydrogen
sulfide can occur under accidental circumstances in industries in
which gas streams with a high hydrogen sulfide content exist.
Furthermore, as hydrogen sulfide is slightly heavier than air, it can
accumulate in toxic concentrations in low-lying areas, even when
generated or leaking at very low rates. However, in such cases, the
environmental levels of hydrogen sulfide have usually only been
measured after the accidents in question, or have been determined by
simulation or reenactment. Concentrations that have been reported
range from 150 mg/m3 (100 ppm), in which a worker lost consciousness
while sawing ebonite boards (Brown, 1969), to 18 000 mg/m3
(12 000 ppm) in a case in which a truck driver died while cleaning the
tank of a vehicle used to transport industrial waste (Simson &
Simpson, 1971). In an outdoor setting, 4 workmen lost consciousness
while digging a pit in marshy land in which hydrogen sulfide
concentrations in air of 442-810 mg/m3 (295-540 ppm) were measured 5
days later (Anonymous, 1952). Alexander (1974) reported hydrogen
sulfide concentrations as high as 0.037 mg/litre (24.8 ppm) in a
sewage stabilization pond and 10.0-13.2 mg/m3 (6.7-8.8 ppm) in the
air 15 m from this pond. In a report by Ahlborg (1951) on hydrogen
sulfide poisoning in the Swedish shale oil industry, concentrations of
hydrogen sulfide measured at various locations in the plant ranged
from 30 to 900 mg/m3 (20-600 ppm), though men seldom worked where
the high levels occurred.
More recently, the US National Institute for Occupational Safety
and Health reported that, in viscose rayon churn rooms, spinning
tanks, and drying and storage cells, workers were mainly exposed
during the working day to hydrogen sulfide concentrations of
23 mg/m3 (15 ppm) or less with occasional peaks of 150 mg/m3
(100 ppm) (NIOSH, 1977).
In the USA, it has been estimated that there are 125 000 employees
potentially exposed to hydrogen sulfide (NIOSH, 1977), Table 1 is a
list of occupations in which such exposure can occur ranging according
to occupation from rare exposure to low concentrations, to frequent
exposure to concentrations very near those associated with adverse
health effects.
Table 1. Examples of occupations with potential exposure to
hydrogen sulfidea
Animal fat and oil processors Lithographers
Animal manure removers Lithopone makers
Artificial-flavour makers Livestock farmers
Asphalt storage workers Manhole and trench workers
Barium carbonate makers Metallurgists
Barium salt makers Miners
Blast furnace workers Natural gas production and
Brewery workers processing workers
Bromide-brine workers Painters using polysulflde
Cable splicers caulking compounds
Caisson workers Papermakers
Carbon disulfide makers Petroleum production and
Cellophane makers refinery workers
Chemical laboratory workers, Phosphate purifiers
teachers, students Photo-engravers
Cistern cleaners Pipeline maintenance workers
Citrus root fumigators Pyrite burners
Coal gasification workers Rayon makers
Coke oven workers Refrigerant makers
Copper-ore sulfidizers Rubber and plastics processors
Depilatory makers Septic tank cleaners
Dyemakers Sewage treatment plant workers
Excavators Sewer workers
Felt makers Sheepdippers
Fermentation process workers Silk makers
Fertilizer makers Slaughterhouse workers
Fishing and fish-processing workers Smelting workers
Fur dressers Soapmakers
Geothermal-power drilling and Sugar beet and cane processors
production workers Sulfur spa workers
Gluemakers Sulfur products processors
Gold-ore workers Synthetic-fibre makers
Heavy-metal precipitators Tank gaugers
Heavy-water manufacturers Tannery workers
Hydrochloric acid purifiers Textile printers
Hydrogen sulfide production Thiophene makers
and sales workers Tunnel workers
Landfill workers Well diggers and cleaners
Lead ore sulfidizers Wool pullers
Lead removers
a From: NIOSH (1977).
5. EFFECTS ON EXPERIMENTAL ANIMALS
Very little information is available on the effects of low level
concentrations of hydrogen sulfide gas on experimental animals; most
published data have emphasized the effects of exposure to lethal or
near-lethal concentrations of the gas. According to Evans (1967) and
Smith & Gosselin (1979), the effects of high doses of hydrogen sulfide
and high doses of cyanide are very similar. Both inhibit the enzyme
cytochrome c oxidase [EC 1.9.3.1]. This was demonstrated in studies
using purified preparations of the enzyme (Smith & Gosselin, 1979).
When sodium sulfide at 0.1, 0.25, and 0.32 mmol/kg body weight was
administered intraperitoneally to mice, sulfide was not exhaled
(Susman et al., 1978) suggesting that it was inactivated primarily by
metabolism. In studies on the inhalation of hydrogen sulfide in rats,
cats, rabbits, and dogs, the nervous centres were first excited arid
then paralysed; pupils first contracted and then dilated; blood
pressure was first raised, then lowered; and respiration first
increased and then halted (Evans, 1967; Haggard, 1925). The findings
of Lehmann (1892) in the cat, dog, and rabbit, of Haggard (1925) in
the dog, and of Sayers et al. (1925) in the canary, rat, guineapig,
dog, and goat, are quite consistent: at 150-225 mg/m3 (100-150 ppm),
signs of local irritation of eyes and throat after many hours of
exposure; at 300-450 mg/m3 (200-300 ppm), eye and mucous membrane
irritation after inhalation for 1 h and slight general effects with
prolonged inhalation; at 750-1050 mg/m3 (500-700 ppm), local
irritation and slight systemic symptoms in less than 1 h and possible
death after several hours' exposure; at 1350 mg/m3 (900 ppm), grave
systemic effects within 30 min and death in less than 1 h; at
2250 mg/m3 (1500 ppm), collapse and death within 15-30 min; and at
2700 mg/m3 (1800 ppm), immediate collapse, respiratory paralysis,
and death.
When mice were exposed repeatedly (4 times for 2 h, at 4-day
intervals) to a hydrogen sulfide concentration in air of 150 mg/m3
(100 ppm), the critical inhibition of terminal cytochrome c oxidase
appeared to be cumulative. This effect was accompanied by a cumulative
decrease in cerebral RNA synthesis (Savolainen et al., 1980).
Experimental studies on rabbits indicated that either a single or
repeated exposure for 1.5 h per day (5 consecutive days) to a hydrogen
sulfide concentration in air of 105 mg/m3 (70 ppm) caused
electrocardiographic (ECG) changes (Kósmider et al., 1967).
Though some small differences in susceptibility to hydrogen
sulfide gas were exhibited among the species studied by Sayers et al.,
(1925), canaries being the most sensitive and goats the most
resistant, the interspecies differences were slight. It is agreed
among investigators that the effects of hydrogen sulfide gas on the
nervous system represent the most important aspect of its toxicity
(Haggard, 1925; Evans, 1967). Beck et al. (1979) showed that ethanol
in doses of 0.33-0.66 g/kg significantly shortened the time to loss of
consciousness in rats exposed to a hydrogen sulfide concentration in
air of 1200 mg/m3 (800 ppm) for 30 min. The induction of
methaemoglobinaemia by the injection of sodium nitrite had both
protective and antidotal effects against hydrogen sulfide poisoning in
mice, armadillos, rabbits, and dogs (Smith & Gosselin, 1979).
Water containing a hydrogen sulfide concentration as low as
0.86 mg/litre was toxic to trout after exposure for 24 h (McKee &
Wolf, 1971).
6. EFFECTS ON MAN
Adequate systematic studies of the relationship between hydrogen
sulfide exposure and health status in the general population have not
been carried out. Controlled exposure of human subjects to
concentrations of hydrogen sulfide gas exceeding about 75 mg/m3
(50 ppm) has been deemed to involve excessive risk because of the
possibility of injury to the lungs (Sayers et al., 1925; National
Research Council, USA, 1979). Furthermore, except for studies related
to odour threshold, controlled exposures of human subjects to very low
concentrations of the gas, for example, below 1.5 mg/m3 (1.0 ppm)
have not been reported. Thus, the information presented in this
section has mainly been derived from reports of accidental and
industrial exposures to hydrogen sulfide. A general discussion of the
toxicology of hydrogen sulfide has been included, because a basic
understanding of the subject is necessary for a discussion of the role
of the gas as an industrial and community hazard.
6.1 General Toxicological Considerations
The following observations have been derived from reports of
studies involving man. However, for clarification, some studies on
experimental animals have also been included. In general, both animals
and man respond in a very similar fashion to toxic concentrations of
hydrogen sulfide. It is both an irritant and an asphyxiant gas
(Table 2) that induces local inflammation of the membranes of the
human eye and respiratory tract (Yant, 1930). It has been shown that
eye irritation, the most commonly reported effect of hydrogen sulfide
exposure, can occur after several hours' exposure to concentrations of
16-32 mg/m3 (10.5-21.0 ppm) (Elkins, 1939; Nesswetha, 1969).
However, pulmonary tract irritation is, potentially, a more serious
reaction. When inhaled by dogs, hydrogen sulfide exerted an irritant
action through the entire respiratory tract, though the deeper
structures suffered the greatest damage (Haggard, 1925). Inflammation
of these deeper structures may result in pulmonary oedema.
Exposure to hydrogen sulfide gas did not induce important effects
on the human skin nor was any appreciable absorption through intact
skin observed (Yant, 1930). However, Petrun (1966) reported that when
the skin of rabbits was exposed to hydrogen sulfide at concentrations
of 1050 and 2100 mg/m3 (700 and 1400 ppm), trace amounts of hydrogen
sulfide were found in the exhaled air of the rabbits. No quantitative
information was given.
Hydrogen sulfide gas is rapidly absorbed through the lung. Like
hydrogen cyanide, it is a potent inhibitor of cytochrome c oxidase
that interferes with tissue use (Smith & Gosselin, 1979). As a result,
the oxidative metabolism may slow to the point where tissue metabolic
demands cannot be met. In the central nervous system, the result may
be paralysis of the respiratory centres. Respiratory arrest and death
from asphyxia would be the natural outcome.
In studies on dogs (Haggard, 1925), hydrogen sulfide at
concentrations of 1500-3000 mg/m3 (1000-2000 ppm) initially
stimulated excessively rapid breathing (hyperpnoea), because of a
depletion in the carbon dioxide content of the blood (hypocapnia).
This was followed by a period of respiratory inactivity (apnoea).
Spontaneous respiration may be reestablished, if carbon dioxide
depletion has not progressed beyond the point where prompt
reaccumulation can act as a stimulus to the reestablishment of
respiration. If spontaneous recovery does not occur and artificial
respiration is not applied rapidly, death from asphyxia is the
inevitable result (Haggard, 1925). At about 2250 mg/m3 (1500 ppm),
the sequence of events in the dog was the same, except that the
reaction was more pronounced; at 3000 mg/m3 (2000 ppm) there was
respiratory paralysis after a breath or two and, in Haggard's words,
"the victim falls to the ground as though struck down". When breathing
ceases, generalized convulsions frequently begin. There appears to be
no clear explanation of the cause of this picture of sudden collapse.
According to Haggard (1925), this form of respiratory failure is not
related to the carbon dioxide content of the blood but, rather, to the
directly paralysing effect of hydrogen sulfide on the respiratory
centre. Breathing is never reestablished spontaneously following this
hydrogen sulfide-induced respiratory paralysis. Haggard noted,
however, that because the heart continues to beat for several minutes
after respiration has ceased, death from asphyxia can be prevented if
artificial respiration is begun immediately and is continued until the
hydrogen sulfide concentration in the blood decreases. This decrease
is probably a consequence of metabolic processes, as shown in mice by
Susman et al. (1978) rather than, as once believed, the result of the
pulmonary excretion of the gas.
Smith & Gosselin (1979) have called attention to the confusion
that exists in the literature with regard to the effects of hydrogen
sulfide on haemoglobin. They emphasize that many studies have proved
that neither sulfhaemoglobin nor any other abnormal pigments are
present in sufficient concentrations in the blood of animals or human
subjects, fatally poisoned by hydrogen sulfide.
The characteristic "rotten egg" odour of hydrogen sulfide is an
important aspect of the toxicology of the gas. The threshold of
perception (odour) varies considerably depending on individual
sensitivity. Several authors have reported odour detection thresholds
ranging from 0.0007 mg/m3 to 0.20 mg/m3 (0.0005-0.13 ppm)
(Table 2). Thus, the odour of hydrogen sulfide gas can be a very
sensitive indicator of its presence in low concentrations. However, at
higher concentrations (> 225 mg/m3 (150 ppm)), hydrogen sulfide
exerts a paralysing effect on the olfactory apparatus (Milby, 1962),
thus neutralizing the value of its odour as a warning signal. Poda
(1966) reported that among 42 workers, who were rendered unconscious
from overexposure to hydrogen sulfide, majority did not smell the
characteristic odour of the gas but noted a sickeningly sweet odour,
very briefly, before losing consciousness.
Table 2. Effects of hydrogen sulfide exposure at various concentrations in air
Concentration
Effect Duration of
mg/m3 ppm exposure Reference
Man
Approximate threshold 0.0007-0.2 0.0005-0.13 A few seconds Yant (1930); Ryazanov
for odour to less (1962); Adams & Young
than 1 min (1968); Leonardos et al.
(1969); Lindvall (1970);
Thiele (1979); Winneke
et al. (1979)
Threshold of eye 16-32 10.5-21 6-7 h Elkins (1939)
irritation Nesswetha (1969)
Acute conjuctivitis 75-150 50-100 > 1 h Yant (1930)
(gas eye)
Loss of sense of smell 225-300 150-200 2-15 min Sayers et al. (1925)
Animalsa
Local irritation and 750-1050 500-700 < 1 h Haggard (1925)
slight systemic symptoms;
possible death
after several hours
Table 2. (Con't)
Concentration
Effect Duration of
mg/m3 ppm exposure Reference
Systemic symptoms; 1350 900 < 30 min Haggard (1925)
death in less than 1 h
Death 2250 1500 15-30 min Haggard (1925)
a These observations were made in experimental animals. However, there are no better quantitative
data available concerning man with respect to exposure to hydrogen sulfide at high concentrations.
Hydrogen sulfide intoxication in man has generally been
categorized according to 3 clinical forms, acute, subacute, and
chronic depending on the nature of the predominant clinical signs and
symptoms (National Research Council, USA, 1979). The term "acute
hydrogen sulfide intoxication" has been most often used to describe
systemic poisoning characterized by rapid onset and predominance of
signs and symptoms of nervous system involvement. The term "subacute
intoxication" has been applied to episodes of poisoning in which signs
and symptoms of eye and respiratory tract irritation were most
prominent. "Chronic hydrogen sulfide intoxication" has been applied by
some authors to describe a prolonged state of symptoms resulting from
a single or repeated exposure to concentrations of hydrogen sulfide
that do not produce clear-cut manifestations of either acute or
subacute illness.
In a document prepared by the National Research Council of the
National Academy of Sciences of the USA (National Research Council,
USA, 1979), the observation was made that application of the terms
"acute", "subacute" and "chronic" to hydrogen sulfide exposure was
both imprecise and misleading. However, rather than abandon these
frequently used terms altogether, the authors suggested a series of
clarifying definitions which are quoted in the following paragraphs,
and will henceforth be used in this section.
" Acute intoxication: Effects of a single exposure [seconds-
minutes]a to massive concentrations of hydrogen sulfide that rapidly
produce signs of respiratory distress. Concentrations approximating
1400 mg/m3 (1000 ppm) are usually required to cause acute
intoxication.
Sub-acute intoxication: Effects of continuous exposure [up to
several hours]a to mid-level 140 to 1400 mg/m3 (100 to 1000 ppm)
concentrations of hydrogen sulfide. Eye irritation (gas eye) is the
most commonly reported effect, but pulmonary edema (in the absence of
acute intoxication) has also been noted.
Chronic intoxication: Effects of intermittent exposures to low
to intermediate concentrations 70 to 140 mg/m3 (50 to 100 ppm) of
hydrogen sulfide, characterized by "lingering", largely subjective
manifestations of illness."
It is important to note that these definitions do not include a
consideration of the health consequences to man associated with
prolonged low-level exposure to hydrogen sulfide gas, such as may be
encountered under conditions of general urban air pollution.
A concentration of hydrogen sulfide in drinking water as low as
0.07 µg/litre (0.05 ppm) can affect its taste (Campbell et al., 1958).
a Time factors added by WHO Task Group.
6.2 Occupational Exposure
In certain occupations, workers are intermittently exposed to
concentrations of hydrogen sulfide that are not only malodorous but
can, in some situations, cause severe adverse health effects and even
death. Usually, hydrogen sulfide is encountered in the workplace as an
undesirable by-product of a manufacturing process, notably petroleum
refining, viscose rayon production, sugar beet processing, and tannery
work (Milby, 1962; NIOSH, 1977). In other occupations, for example
cesspool cleaning and work in sewers, exposure to hydrogen sulfide may
occur when the gas is formed as a result of the decomposition of
sulfur-containing organic matter, in the absence of complete
oxidation. Deaths attributed to such exposure occurred in the sewers
of Paris in the 1780s (Mitchell & Davenport, 1924) and still occur
under various circumstances.
Workers in certain occupations risk exposure to naturally
occurring hydrogen sulfide; geothermal energy workers and spa
attendants may be included in this category.
Acute hydrogen sulfide intoxication is a dramatic, often fatal
event. Three men were inadvertently enveloped in a cloud of hydrogen
sulfide gas escaping from a cylinder under high pressure; all fell, as
if struck down, and ceased breathing. Only as a result of prompt
resuscitation by trained onlookers did the men survive, though the two
most seriously affected experienced violent convulsions and did not
recover consciousness for some 30 min. None of the men suffered
important after-effects, and none recalled having noted the
characteristic odour of hydrogen sulfide. The hydrogen sulfide
concentrations to which the men were exposed were estimated to be
about 2800 mg/m3 (2000 ppm) (Milby, 1962). Twelve workmen in a plant
that produced benzyl polysulfide were overcome by hydrogen sulfide
gas, when a pipe used to transfer sodium sulfhydrate ruptured. The
liquid sulfhydrate drained into a nearby sewer, where it reacted with
acid sewage releasing hydrogen sulfide from several sewer openings in
the immediate vicinity. Two of the 12 workmen died, probably as a
result of respiratory arrest; 3 stopped breathing but were
successfully resuscitated; 6 lost consciousness but recovered
spontaneously, and 1 individual developed pulmonary oedema that
responded to therapy (Kleinfeld et al., 1964).
Burnett et al. (1977) reviewed 221 cases of exposure to hydrogen
sulfide associated with the oil, gas, and petrochemical industries in
Canada. The overall mortality was 6%; three-quarters of all victims
experienced a period of unconsciousness and 12% were comatose. A high
proportion of patients had other neurological signs and symptoms,
including altered behaviour patterns, confusion, vertigo, agitation,
or somnolence. Respiratory tract effects were second in frequency only
to neurological manifestations. Forty percent of all cases required
some form of respiratory assistance and 15% of all cases developed
pulmonary oedema. Less severely affected patients complained primarily
of headaches, sore eyes, or gastrointestinal upsets. There were no
recognizable sequelae among the survivors. Data on environmental
exposure levels were not reported.
Nearly fatal cases of acute hydrogen sulfide intoxication
associated with sequelae of varying severity have been reported. A
48-year-old farmer, who collapsed from hydrogen sulfide intoxication
while shovelling manure, continued to have convulsive seizures after
resuscitation. The ECG changes suggested myocardial infarction. The
patient recovered but a slight, persistent dizziness remained
(Kaipainen, 1954). A 46-year-old sewer worker, who was overcome by
hydrogen sulfide in a manhole for 30 min, was cyanotic, suffering
generalized spasms, and required artificial respiration. A week later,
he could move and speak only with great effort; a month afterwards, he
still exhibited neurological deficits. The ECG showed evidence of
small anterolateral infarct and a right bundle branch block. Three
months later, although ambulatory, the patient still suffered from
anginal pain upon exertion (Hurwitz & Taylor, 1954). In a man who had
suffered severe hydrogen sulfide intoxication with collapse and
respiratory failure, ECG evidence of myocardial ischaemia was noted
during the early phase of acute illness but gradually disappeared over
a period of 15 days (Kemper, 1966). Each of these 3 events, in which
sequelae were reported, were characterized by periods of
unconsciousness during which hypoxia of vital issues was likely to
have occurred and may have been the basis for the observed prolonged
effects. Many other instances of sequelae following acute hydrogen
sulfide intoxication have been reported. Most resemble the cases just
mentioned, in which serious poisoning with unconsciousness preceded
the appearance of sequelae.
Ahlborg (1951) described 58 cases of acute hydrogen sulfide
intoxication in Sweden's shale oil industry. There were no fatalities.
Symptoms were generally uniform: sudden fatigue, dizziness, and
intense anxiety followed by unconsciousness with or without
respiratory failure. Several cases of acute poisoning were not
associated with unconsciousness, otherwise symptoms were similar to
those of the other cases. The diagnosis of "sequelae after acute
hydrogen sulfide poisoning" was made in 15 cases. The majority of
these had a history of repeated acute intoxications followed in each
case by neurasthenic problems (fatigue, somnolence, headache, lack of
initiative, irritability, anxiety and poor memory, and decreased
libido), though some developed sequelae following acute intoxication
without an intercurrent episode of unconsciousness. Many stricken
workers developed an increase in sensitivity (aversion) to the odour
of gas of any type, even pure gasoline vapour (Ahlborg, 1951).
Numerous case histories of fatal hydrogen sulfide intoxication
have been reported (Larson et al., 1964; Adelson & Sunshine, 1966;
Simson & Simpson, 1971). Oedema of the lungs and brain are common
post-mortem findings. The presence of detectable concentrations of
hydrogen sulfide in the blood has been reported on several occasions
(Larson et al., 1964; Adelson & Sunshine, 1966) and concentrations in
the blood of fatally poisoned victims have ranged from 1.70 mg to
3.75 mg/litre (McAnalley et al., 1979).
In acute hydrogen sulfide intoxication, cessation of respiration
is an immediate threat to life. Accordingly, the provision of
artificially assisted respiration on an emergency basis is absolutely
critical. There is some question as to whether mouth-to-mouth
resuscitation may create a potential health hazard to the rescuer,
because of the presence of hydrogen sulfide in the expired air or on
the clothing of the victim. Thus, methods of artificial respiration
requiring less direct contact (for example, back-pressure-arm lift)
may be prudent.
6.3 General Population Exposure
There are several reports of episodes of general population
response to air contamination by hydrogen sulfide. Information derived
from these events is consistent with observations reported among
workers occupationally exposed to hydrogen sulfide. Table 2
demonstrates the wide range of odour perception thresholds for
hydrogen sulfide reported by various investigators. In view of the
magnitude of these differences, it is not possible to state with
certainty the concentration at which odour-related complaints can be
expected.
A catastrophic exposure episode involving the release of large
quantities of hydrogen sulfide into a small community was reported by
McCabe & Clayton (1952). This occurred in 1950 in Poza Rica, Mexico, a
city of 22 000 people located about 210 km northeast of Mexico City.
Poza Rica was then the centre of Mexico's leading oil-producing
district and the site of several oil field installations, including a
sulfur-recovery plant. An early morning malfunction of the waste gas
flare resulted in the release of large quantities of unburned hydrogen
sulfide into the atmosphere. The unburned gas, aided by a low-level
temperature inversion and light early morning breezes, was carried to
a residential area adjacent to the plant area. Residents of the area
were overcome while attempting to leave the area and while assisting
stricken neighbours. Within 3 h, 320 persons were hospitalized and 22
died. The most frequent symptom was loss of the sense of smell. More
than half of the patients lost consciousness, many suffered signs and
symptoms of respiratory tract and eye irritation and 9 developed
pulmonary oedema. Four of the 320 victims exhibited neurological
sequelae; 2 experienced neuritis of the acoustic nerve; 1 developed
dysarthria; the fourth patient suffered aggravation of pre-existing
epilepsy. The duration of these neurological sequelae was not
reported.
There have been reports of other episodes of general atmospheric
pollution by hydrogen sulfide evolved from both natural and industrial
sources, but none has been as severe as the Poza Rica incident.
In the geothermal areas of Rotorua, New Zealand, a city of 40 000
people, steam and hot water from approximately 500 active bores are
used to heat houses, buildings, and swimming pools, and to provide
domestic hot water supplies and steam for cooking boxes.
In these areas, accidental fatal cases of acute hydrogen sulfide
intoxication associated with improper ventilation of geothermal steam-
heated dwellings have occasionally been reported. For example, at
least 3 deaths from acute hydrogen sulfide poisoning occurred in 1962.
However, with the introduction of legal requirements regarding the
safe domestic use of the geothermal resources, and possibly also as a
result of increased public awareness of the dangers involved, no
fatalities attributable to hydrogen sulfide have occurred in Rotorua
since 1962 (Thom & Douglas, 1976).
Air pollution monitoring for hydrogen sulfide has been conducted,
during several periods over the past 15 years, at various sites in
Rotorua. However, there has been considerable variation in the results
obtained between the various sites, and furthermore, the
concentrations measured showed considerable seasonal variations. Some
of the results of these measurements are mentioned in section 4.1.
In 1964, the Division of Air Pollution, US Public Health Service,
reported that in the city of Terre Haute, Indiana, biodegradation of
industrial wastes in a 14.5-ha lagoon caused the atmospheric
concentration of hydrogen sulfide to reach a 1-h mean concentration of
0.45 mg/m3 (0.3 ppm). As a result, 81 complaints were registered by
the public, 41 of which were health-related. The most common
complaints were concerned with the perception of a foul odour. The
most common effects were nausea, interruption of sleep, burning of the
eyes, and shortness of breath. Less common manifestations were cough,
headache, and anorexia. Several acute asthma attacks were reported,
but the association of these attacks with the hydrogen sulfide
incident was not clearly established. Though no grave physical
illnesses could be directly related to the air pollution incident, the
investigators stressed that the odours emanating from the lagoon
caused more than a mere nuisance (United States Public Health Service,
1964).
The Poza Rica tragedy provides ample evidence that the accidental
release of hydrogen sulfide into a community can be expected to cause
systemic intoxication of varying severity. The Rotorua experience is
notable in that it emphasizes the fact that the potential for serious,
even fatal, hydrogen sulfide intoxication is present in active
geothermal areas. The more common picture of general population
exposure is exemplified by the Terre Haute incident where an industry-
related source of low-level concentrations of hydrogen sulfide gas
created a health problem which, although not grave, exceeded the level
of mere nuisance.
The potential of long-term, low-level exposure to hydrogen sulfide
to cause pulmonary changes of the type known to be associated with
other irritant gases such as oxides of nitrogen and sulfur has
scarcely been studied. No epidemiological data are available, at
present, upon which to base any sound conclusions.
7. EVALUATION OF HEALTH RISKS TO MAN FROM EXPOSURE TO HYDROGEN SULFIDE
By far the most important recognized toxic effect of hydrogen
sulfide is its ability to induce acute intoxication, characterized by
immediate collapse, frequently accompanied by respiratory arrest and,
without treatment, death. The scientific literature abounds with cases
of this type, most often associated with industrial overexposure.
However, a few cases of acute hydrogen sulfide exposure have been
recorded in the general population as a result of the release of
hydrogen sulfide either from an industrial process or from natural
sources. A second form of injury associated with exposure to hydrogen
sulfide is caused by the irritative action of the gas on the mucous
membranes of the eyes and respiratory tract. Keratoconjunctivitis (gas
eye) and pulmonary oedema are two most serious manifestations of this
local irritative effect. The malodorous property of hydrogen sulfide
gas is well recognized and this characteristic alone is believed by
many to be capable of producing impairment of human health and well-
being.
Most of the information available on human health effects
associated with exposure to various concentrations of hydrogen sulfide
gas has come from observations on accidental and industrial exposures.
With the exception of the Poza Rica catastrophe, information on
general population exposures and associated health effects is sketchy
at best. There is also little information on controlled human exposure
to hydrogen sulfide gas, and, except for data from odour threshold
studies, the information that is available is more than 50 years old.
Although a small amount of information is available on the effects on
experimental animals of high concentrations of hydrogen sulfide gas,
there is virtually no information on the long-term, low-level effects
of the gas on experimental animals. Furthermore, epidemiological data
are lacking concerning the health consequences of long-term, low-level
exposure to hydrogen sulfide, in both the general and industrial
populations.
7.1 Exposure Levels
General population air pollution problems associated with hydrogen
sulfide arise mainly in connexion with malodorous conditions,
traceable to point sources. Such sources can be industrial or, in some
cases, polluted bodies of walter. Peak levels as high as 0.20 mg/m3
(0.13 ppm) have been reported in the air in the neighbourhood of
industrial sources. Hydrogen sulfide is also a common pollutant in
geothermally active areas. At one site is a geothermal area in New
Zealand, where continuous measurements were carried out over a 5-month
period, a level of 0.08 mg/m3 (0.05 ppm) was exceeded, on average,
for 35% of the time.
Concentrations of hydrogen sulfide in the workplace also vary
widely. In the shale oil industry and in viscose rayon production, for
example, maximum levels of exposure during the work day have been
reported to range from 23 to 30 mg/m3 (15-20 ppm). In general,
however, massive accidental exposure to hydrogen sulfide has
constituted the principal hazard of this gas in industrial settings.
In many cases, such exposure has occurred because of equipment
breakage or malfunction. However, because hydrogen sulfide is heavier
than air, it can accumulate in lethal concentrations in low-lying or
enclosed areas. Numerous fatalities have occurred from the slow,
insidious accumulation of the gas in the air in both ambient and
industrial environments.
7.2 Experimental Animal Studies
The toxic effects of hydrogen sulfide gas have not been studied
extensively in experimental animals. However, in studies on a number
of animal species including the mouse, rat, cat, dog, and goat, it has
been shown that the primary target of hydrogen sulfide in high doses
is the nervous system. Collapse, followed by respiratory arrest and
asphyxia resulting from the paralysing effects of high concentrations
of hydrogen sulfide on the respiratory centres of the central nervous
system, is the usual sequence of events leading to death.
Little information is available in the published literature
concerning the effects in experimental animals of long-term, low-level
exposure to hydrogen sulfide.
7.3 Effects of Occupational Exposure
Inadvertent and accidental exposure of human subjects to high
concentrations of hydrogen sulfide has occurred among workers engaged
in petroleum refining, viscose rayon production, sugar beet
processing, and tannery work. Exposure levels have not been precisely
documented in many of these situations. Reported effects range from
the relatively less grave conditions of neurasthenic and
otoneurological symptoms and keratoconjunctivitis to the more serious
effects of pulmonary oedema, respiratory failure, collapse, and even
death. From the available data, it can be estimated that exposure for
seconds or minutes to concentrations of approximately 1400 mg/m3
(1000 ppm) or more would cause acute intoxication, concentrations of
140-1400 mg/m3 (100-1000 ppm) with an exposure time of up to several
hours would produce keratoconjunctivitis and pulmonary oedema, and
intermittent exposures to concentrations of 70-140 mg/m3
(50-100 ppm) could be associated with lingering, largely subjective,
manifestations believed by some to represent chronic intoxication.
Various studies have associated exposure to hydrogen sulfide in
concentrations as low as 16-32 mg/m3 (10.5-21.0 ppm) for several
hours with eye irritation in workers.
Effects of low-level, long-term industrial exposure to hydrogen
sulfide have not been systematically evaluated.
7.4. Effects of General Population Exposure
Several episodes of exposure of the general population to hydrogen
sulfide emanating from a specific source have been investigated and
described. For the most part, these events involved only annoyance
because of the odour or, at worst, minor temporary illness such as
headache, nausea, and sleeplessness. However, as described in section
6.3, on two occasions, general population exposure to hydrogen sulfide
caused grave illness and even death: one at Poza Rica, Mexico, and the
other in and around Rotorua, New Zealand.
Unfortunately, these incidents were not studied using
epidemiological techniques, and it is not possible to establish
exposure-effect relationships from the data.
7.5 Guidelines for the Protection of Public Health
There are two aspects concerning the protection of public health
in relation to hydrogen sulfide exposure: (a) the protection of the
public, and occupational groups in particular, from the toxicological
effects of such exposure; and (b) the protection of the public from
the odour nuisance that can be associated with releases of hydrogen
sulfide.
The odour threshold for hydrogen sulfide has been variously
reported to range from 0.0007 mg to 0.20 mg/m3 (0.0005-0.13 ppm)
(Table 2). Little information is available on the odour detection
limits for ambient hydrogen sulfide either under experimental field
conditions or in general population exposures. The Task Group
considered that a level of 0.007 mg/m3 (0.005 ppm) averaged over
30 min should not produce odour nuisance in most situations. Some
regulatory bodies may wish to adopt longer averaging times with
appropriately adjusted concentration limits.
The best estimates available suggest that eye irritation may occur
in man after several hours' exposure to hydrogen sulfide
concentrations of 16-32 mg/m3 (10.5-21.0 ppm). As occupational
exposure guidelines, the Task Group recommended the adoption of
10 mg/m3 (7 ppm) as a workshift time-weighted average value together
with a short-term exposure limit of 15 mg/m3 (10 ppm). The short-
term limit should be determined as a 10-min or less averaged value.
These limits should prevent eye irritation in workers which represents
the earliest recognized toxic response in man.
Annex tables 1 and 2 contain various national standards or
recommendations for ambient air quality and occupational exposure
limits for hydrogen sulfide. As can be seen, there is considerable
consensus regarding occupational exposure limits and the
recommendations of the Task Group are generally consistent with the
national values. There is less agreement regarding ambient air quality
standards, possibly because of different values placed on the nuisance
value of odours.
REFERENCES
ADAMS, D. F., YOUNG, F. A., & LUHR, R. A. (1968) Evaluation of an odor
perception threshold test facility. Tappi, 51: 62A-67A.
ADELSON, L. & SUNSHINE, I. (1966) Fatal hydrogen sulfide intoxication.
Report of three cases occurring in a sewer. Arch. Pathol.,
81: 375-380.
AHLBORG, G. (1951) Hydrogen sulfide poisoning in shale oil industry.
Am. Med. Assoc. ind. Hyg. occup. Med., 3: 247-266.
AIHA (1965) Hydrogen sulfide. Anal. Abstr., 12: 2207.
ALEXANDER, M. (1974) Microbial formation of environmental pollutants.
Adv. appl. Microbiol., 18: 37-40.
AMERICAN CONFERENCE OF GOVERNMENTAL INDUSTRIAL HYGIENISTS (1972)
Air sampling instruments for evaluation of atmospheric
contaminants, 4th ed., Cincinnati, ACGIH.
ANDERSSON, G., BROSSET, C., & GRENNFELT, P. (1974) The stability of
emitted odorous compounds in the atmosphere. In: Turk, A.,
Johnston, J. W. Jr, & Moulton, D. G., ed. Human response to
environmental odors, New York, Academic Press.
ANONYMOUS (1952) Four workers overcome by hydrogen sulfide when
digging in marshy land. Occup. Health, 12: 39.
BAMESBERGER, W. L. & ADAMS, D. F. (1969) Improvements in the
collection of hydrogen sulfide in cadmium hydroxide suspension.
Environ. Sci. Technol., 3: 258-281.
BAMESBERGER, W. L. & ADAMS, D. F. (1969) Field comparison of the
coulometric, colorimetric, and lead acetate tape analysis methods
for sulfur-containing gases. Tappi, 52: 1302-1306.
BEASLEY, R. W. R. (1963) The eye and hydrogen sulfide. Br. J. ind.
Med., 20: 32-34.
BECK, J. F., CORMIER, F. & DONINI, J. C. (1979) The combined toxicity
of ethanol and hydrogen sulfide. Toxicol. Lett., 3: 311-313.
BECKER, W. J. (1979) On H2S emission and immission measuring
techniques. Staub-Reinhalt. Luft, 39: 169-174.
BERUASHVILI, S. A. (1977) Skin irritant effect of water containing
hydrogen sulfide and results of population studies. In: Annual
Report of the Natadse Institute of Sanitary and Hygiene. Tbilisi,
Vol. XII, pp. 60-63.
BERUASHVILI, S. A. (1979) The long-term effects of the thermal water,
used for the hot water-supply. In: Proceedings of the 4
Conferences of the Young Scientists and Specialists on the
Problem "Environmental Health", Baku, pp. 3-5.
BLACK, M. S., HERBST, R. P., & HITCHCOCK, D. R. (1978) Solid absorbent
preconcentration and gas chromatographic analysis of sulfur gases.
Anal. Chem., 50: 848-851.
BROWN, K. E. (1969) Some toxicological problems in a rubber industry.
Med. J. Aust., 1: 534-538.
BURNETT, W. W., KING, E. G., GRACE, M., & HALL, W. F. (1977) Hydrogen
sulfide poisoning: review of 5 years' experience. Can. Med.
Assoc. J., 117: 1277-1280.
CALIFORNIA AIR RESOURCES BOARD (1970) Ambient air quality standards,
Sacramento, California, California Air Resources Board, pp. 69-75.
CAMPBELL, C. L., DAWES, R. K., DEOLALKAR, S., & MERRITT, M. C. (1958)
Effect of certain chemicals in water on the flavor of brewed
coffee. Food Res., 23: 575-579.
COOPER, R. C., JENKINS, D., & YOUNG, L. Y. (1976) Aquatic
microbiology laboratory manual, Texas, University of Texas.
DENMEAD, C. F. (1962) Air pollution by hydrogen sulfide from a shallow
polluted tidal inlet, Auckland, New Zealand. In: Clean Air
Conference, Sydney, Australia.
ELKINS, H. B. (1939) Toxic fumes. Ind. Med., 8: 426-432.
ESPACH, R. H. (1950) Sources of hydrogen sulfide in Wyoming. Ind.
Eng. Chem., 42: 2235-2237.
EVANS, C. L. (1967) The toxicity of hydrogen sulfide and other
sulfides. Quart. J exp. Physiol., 52: 231-248.
FEDERAL MINISTER FOR HOME AFFAIRS (1974) [Technical instructions for
prevention of air pollution, issued 28 August 1974]. pp. 175
(in German).
FUKUI, S., NAITO, S., KANEKO, M., & KANNO, S. (1967) [Hygienic
chemical studies on public nuisance by injurious gases. X.
Improvement of absorption mixture and examination of the methylene
blue method for determination of hydrogen sulfide.] Hyg. Chem.,
13: 16-21 (in Japanese).
GILARDI, E. F. & MANGANELLI, R. M. (1963) A laboratory study of a lead
acetate-tile method for the quantitative measurement of low
concentrations of hydrogen sulfide. Air Pollut. Control Assoc.,
13: 305-309.
GOLDMAN, F. H., COLEMAN, A. L., ELKINS, H. B., SCHRENK, H. H., &
SMUCKER, C. A. (1940) II. Report of sub-committee on chemical
methods in air analysis. Sampling and sampling devices.
Am. public Health Assoc. Year Book, pp. 92-98.
GOLDMAN, F. H., COLEMAN, A. A., ELKINS, H. B., & SCHRENK, H. H. (1943)
II. Report of sub-committee on chemical methods in air analysis.
Cadmium and hydrogen sulfide. Am. J. public Health, 33: 862-864.
GRANAT, L., RODHE, H., & HALLBERG, R. O. (1976) The global sulfur
cycle. In: Svensson, B. H. & Söderlund, R., ed. Nitrogen,
phosphorus and sulfur -- global cycles, SCOPE report 7. Ecol.
Bull. (Stockholm), 22: 89-134.
HAGGARD, H. W. (1925) The toxicology of hydrogen sulfide. J. ind.
Hyg., 7: 113-121.
HURWITZ, L. J. & TAYLOR, G.I. (1954) Poisoning by sewer gas with
unusual sequelae. Lancet, 1: 1110-1112.
ILO (1971) Tanning, leather finishing. In: Encyclopedia of
Occupational Health and Safety, 2nd ed., Geneva, International
Labour Office, pp. 1381-1382.
ILO (1971-72) Hydrogen sulfide. In: Encyclopedia of Occupational
Health and Safety, 2nd ed., Geneva, International Labour Office,
pp. 697-699.
INSTITUTE OF HYGIENE & EPIDEMIOLOGY (1978) [Air pollution by H2 S
at Boom, Belgium.] Brussels, pp. 1-17 (L.V.M. BO.77) (in Dutch).
ISO (1978) Proposal to the SC3 for the determination of hydrogen
sulfide in air, Geneva, International Organization for
Standardization, 8 pp. (ISO/TC 146/SC3/WG3 N 2E (revised),
1978-06-14).
INTERSOCIETY COMMITTEE (1977 a) Tentative method of analysis for
hydrogen sulfide content of the atmosphere. In: Katz, M., ed.
Methods of air sampling & analysis, 2nd ed., pp. 676-681.
INTERSOCIETY COMMITTEE (1977 b) Tentative method of gas
chromatographic analysis for sulfur-containing gases in the
atmosphere (automatic method with flame photometer detector).
In: Katz, M., ed. Methods of air sampling & analysis, 2nd ed.,
pp. 722-737.
JACOBS, M. B. (1965) Recommended standard method for continuing air
monitoring for hydrogen sulfide. Ultramicrodetermination of
sulfides in the air. J. Air Pollut. Control Assoc., 15: 314-315.
JOHNSON, B. A. (1972) The evaluation of gas detector tube systems:
hydrogen sulfide. Am. Ind. Hyg. Assoc. J., 33: 811-812.
JUNGE, C. E. (1963) Air chemistry and radioactivity, New York,
Academic press, pp. 68-69 (International Geophysics series
Vol. 4).
KAIPAINEN, W. J. (1954) Hydrogen sulfide intoxication. Rapidly
transient changes in the electrocardiogram suggestive of
myocardial infarction. Ann. Med. intern. Fenn., 43: 97-101.
KATZ, M. (1977) Sulfur and its inorganic derivatives in the Canadian
environment, Ottawa, Associate Committee on Scientific Criteria
for Environmental Quality, National Research Council of Canada,
pp. 21-68 (Publ. No. NRCC 15015).
KELLOGG, W. W., CADLE, R. D., ALLEN, E. R., LAZRUS, A. L., & MARTELL,
E. A. (1972) The sulfur cycle. Man's contribution as compared to
natural sources of sulfur compounds in the atmosphere and oceans.
Science, 175: 587-596.
KEMPER, F. D. (1966) A near-fatal case of hydrogen sulfide poisoning.
Can. Med. Assoc. J., 94: 1130-1131.
KLEINFELD, M., GIEL, C., & ROSSO, A. (1964) Acute hydrogen sulfide
intoxication; an unusual source of exposure. Ind. Med. Surg.,
33: 656-660.
KOSMIDER, S., ROGALA, E., & PACHOLEK, A. (1967) Electrocardiographic
and histochemical studies of the heart muscle in acute
experimental hydrogen sulfide poisoning. Arch. Immunol. ther.
Exp., 15: 731-740.
KRASOVITSKAYA, M. L., MALJAROVA, L. K. & ZAPOROZEC, T. S. (1965)
[Contamination of the air in the area of a refinery and
petrochemical plant.] Gig. i Sanit., 4: 103-105 (in Russian).
LARSON, C. P., REBERGER, C. C., & WICKS, M. J. (1964) The purple brain
death. Med. Sci. Law, 4: 200-202.
LAWRENCE BERKELEY LABORATORY (1976) Instrumentation for environmental
monitoring: air pollution. Ambient air monitor, Multi-component
monitoring system for air pollution and SO2 monitor, California,
University of California.
LEHMANN, K. B. (1892) [Experimental studies on the effect on the body
of gases and vapours of technical and hygienic importance, Part V,
hydrogen sulfide.] Arch. Hyg., 14: 135-189 (in German).
LEICHNITZ, K. (1977) Air analyses by means of long-term detector
tubes. Dräger Rev., 40: 9-17.
LEONARDOS, G., KENDALL, D., & BARNARD, N. (1969) Odor threshold
determination of 53 odorant chemicals. J. Air Pollut. Control
Assoc., 19: 91-95.
LEVAGGI, D. A., SIU, W., & FELDSTEIN, M. (1972) Continuous
determination of H2S in the PPB range by a specific colorimetric
method. In: Proceedings of the Technicians International
Congress, N.Y., pp. 65-67.
LINDVALL, T. (1970) On sensory evaluation of odorous air pollutant
intensities. Measurements of odor intensity in the laboratory and
in the field with special reference to effluents of sulfate pulp
factories. Nord. Hyg. Tidskr., 51 (suppl.): 36-39.
MACALUSO, P. (1969) Hydrogen sulfide. In: Mark, H. F., McKetta, J. J.,
& Othmer, D. F., ed. Encyclopedia of chemical technology, 2nd
ed., N.Y., John Wiley & Sons.
McANALLEY, B. H., LOWRY, W. T., OLIVER, R. D., & GARRIOTT, J. C.
(1979) Determination of inorganic sulfide and cyanide in blood
using specific ion electrodes: application to the investigation of
hydrogen sulfide and cyanide poisoning. J. anal. Toxicol.,
3: 111-114.
McCABE, L. C. & CLAYTON, G. D. (1952) Air pollution by hydrogen
sulfide in Poza Rica, Mexico. An evaluation of the incident of
Nov. 24, 1950. Am. Med. Assoc. Arch. ind. Hyg. occup. Med.,
6: 199-213.
McGRAW-HILL ENCYCLOPEDIA OF SCIENCE & TECHNOLOGY (1960) Heavy water,
New York, McGraw-Hill Book Co. Inc., pp. 382-383.
McKEE, J. E. & WOLF, H. W. (ed.) (1971) Water quality criteria, 2nd
ed., California, The Resources Agency of California, State Water
Resources Control Board, 584 pp. (Publ. No. 3-A).
MERCADO, S. G. (1975) Geothermo-electric project of Cerro-Prieto:
contamination and basic protection. In: Proceedings of the Second
UN Symposium on the Development and Use of Geothermal Resources,
San Francisco, CA, pp. 1385-1393.
MILBY, T. H. (1962) Hydrogen sulfide intoxication. Review of the
literature and report of unusual accident resulting in two cases
of nonfatal poisoning. J. occup. Med., 4: 431-437.
MINER, S. (1969) Preliminary air pollution survey of hydrogen
sulfide. A literature review, Raleigh, NC, National air
pollution control adm. (Publ. No. APTD 69-37).
MINSTER, J. T. (1963) Meteorology. Atmospheric hydrogen sulfide
concentrations during the London fog of December 1962. Nature
(Lond.), 199: 474-475.
MITCHELL, C. W. & DAVENPORT, S. J. (1924) Hydrogen sulfide literature.
Public health Rep., 39 (1): 1-13.
NATIONAL RESEARCH COUNCIL, USA (1979) Subcommittee on hydrogen
sulfide. Hydrogen sulfide, Baltimore, University Park Press.
NATUSCH, D. F. S., SEWELL, J. R., & TANNER, R. L. (1974) Determination
of hydrogen sulfide in air -- an assessment of impregnated paper
tape methods. Anal. Chem., 46 (3): 410-415.
NESSWETHA, W. (1969) [Eye lesions caused by sulfur compounds.]
Arbeitsmed. Sozialmed. Arbeitshyg., 4:288-290 (in German).
NEWILL, V. A. (1977) Air quality standards. In: Stern, A. D., ed. Air
pollution, Vol. V, Air quality management, 3rd ed., New York,
Academic Press.
NIOSH (1977) Occupational exposure to hydrogen sulfide, Cincinnati,
OH, National Institute for Occupational Safety and Health (DHEW
(NIOSH) publ. No. 77-158).
NYMAN, H. Th. (1954) Hydrogen sulfide eye inflammation -- treatment
with cortisone. Ind. Med. Surg., 23: 161-162.
PARÉ, J. P. (1966) A new tape reagent for the determination of
hydrogen sulfide in air. J. Air Pollut. Control Assoc.,
16: 325-327.
PEREGUD, Y. A., GERNET, Y. V., & BYKHOOSKAYA, M. S. (1971) Rapid
methods for the determination of noxious substances in the air,
Springfield, VA, US Dept. of Commerce, National Technical
Information Service, (AD 126795).
PETRUN, N. M. (1966) [Penetration of hydrogen sulfide through skin and
its influence on gaseous exchange and energy metabolism.] In:
Pharmacol. Toxicol., 2nd ed., Kiev, Publishing House "Health"
("Zdorovie"), pp. 247-250 (in Russian).
PODA, G. A. (1966) Hydrogen sulfide can be handled safely. Arch.
environ. Health, 12: 795-800.
ROBINSON, E. & ROBBINS, R. C. (1970) Gaseous sulfur pollutants from
urban and natural sources. J. Air Pollut. Control Assoc.,
20: 233-235.
ROLFE, K. A. (1980) The air-pollution aspects of geothermal power
stations. N.Z. energy J., 53: 51-58.
RYAZANOV, V. A. (1962) Sensory physiology as basis for air quality
standards. Arch. environ. Health, 5: 480-494.
SANDERSON, H. P., THOMAS, R., & KATZ, M. (1966) Limitations of the
lead acetate impregnated paper tape method for hydrogen sulfide.
J. Air Pollut. Control Assoc., 16: 328-330.
SAVOLAINEN, H., TENHUNEN, R., ELOVAARA, R., & TOSSAVAINEN, A. (1980)
Cumulative biochemical effects of repeated subclinical hydrogen
sulfide intoxication in mouse brain. Int. Arch. occup. environ.
Health, 46: 87-92.
SAYERS, R. R., SMITH, N. A. C., FIELDNER, A. C., MITCHELL, C. W.,
JONES, G. W., YANT, W. P., STARK, D. D., KATZ, S. H., BLOOMFIELD,
J. J., & JACOBS, W. A. (1925) Investigation of toxic gases from
Mexican and other high-sulfur petroleums and products. Report by
the Dept. of the Interior, Bureau of Mines, to the American
Petroleum Institute, Washington, DC, US Government Printing
Office, pp. 59-80 (Bull. No. 231).
SHAW, J. A. (1940) Rapid determination of hydrogen sulfide and
mercaptan sulfur. In gases and in aqueous solutions. Anal. Chem.,
12: 668-671.
SIMSON, R. E. & SIMPSON, G. R. (1971) Fatal hydrogen sulfide poisoning
associated with industrial waste exposure. Med. J. Aust.,
1: 331-334.
SIU, W., LEVAGGI, D. A., POTTER, L., MARTIN, R., & FELDSTEIN, M.
(1971) Modifications to an H2S tape sampler for increasing
sensitivity and accuracy in H2S sampling. J. Air Pollut.
Control Assoc., 21 (10): 636-638.
SMITH, R. P. & GOSSELIN, R. E. (1979) Hydrogen sulfide poisoning.
J. occup. Med., 21: 93-97.
STEVENS, R. K., MULIK, J. D., O'KEEFFE, A. E., & KROST, K. J. (1971)
Gas chromatography of reactive sulfur gases in air at the parts-
per-billion level. Anal. Chem., 43: 827-831.
STINE, R. J., SLOSBERG, B., & BEACHAM, B. E. (1976) Hydrogen sulfide
intoxication. A case report and discussion of treatment. Ann.
intern. Med., 85 (6): 756-758.
SUSMAN, J. L., HORNIG, J. F., THOMAE, S. C., & SMITH, R. P. (1978)
Pulmonary excretion of hydrogen sulfide, methanethiol, dimethyl
sulfide and dimethyl disulfide in mice. Drug Chem. Toxicol.,
1: 327-338.
THIELE, V. von (1979) [Experimental investigations to determine an
odour threshold value for hydrogen sulfide.] Staub-Reinhalt.
Luft, 39: 159-160 (in German).
THOM, N. G. & DOUGLAS, R. T. (1976) A study of hydrogen sulfide levels
in the geothermal areas of Rotorua, New Zealand. In: Fourth
International Clean Air Congress, Tokyo, pp. 565-568.
THOMPKINS, F. C., Jr & BECKER, J. H. (1976) An evaluation of
portable, direct-reading H2 S meters, Research Report,
Cincinnati, OH, National Institute for Occupational Safety and
Health (DHEW (NIOSH) Publ. No. 77-137).
UNITED STATES PUBLIC HEALTH SERVICE (1941) Hydrogen sulfide: its
toxicity and potential dangers, Cincinnati, OH, US Dept of
Health, Education, and Welfare, Public Health Service, Division of
Air Pollution, pp. 684-693.
UNITED STATES PUBLIC HEALTH SERVICE (1964) The air pollution
situation in Terre Haute, Indiana with special reference to the
hydrogen sulfide incident of May - June, 1964, Cincinnati, OH,
US Dept of Health, Education, and Welfare, Public Health Service
Division of Air Pollution, pp. 1-28.
WEAST, R. C., ed. (1977-78) Physical constants of inorganic compounds.
In: Handbook of chemistry and physics, 58th ed., Cleveland, OH,
USA, CRC Press.
WEST, P. W. (1970) Analytical methods for the study of air pollution.
Pure appl. Chem., 21: 437-447.
WINDHOLZ, M., ed. (1976) Hydrogen sulfide. In: Merck index, 9th ed.,
Rahway, N. J., Merck & Co. Inc., pp. 633-634.
WINNEKE, VON G., KOTALIK, J., KELDENICH, H.-O., & KASTKA, J. (1979)
[The identification of hydrogen sulfide under laboratory and field
conditions.] Staub-Reinhalt. Luft, 39:156-159 (in German).
YANT, W. P. (1930) Hydrogen sulfide in industry, occurrence, effects,
and treatment. Am. J. public Health, 20: 598-608.
Annex
Annex table 1. Ambient air quality standards for hydrogen sulfide from selected countries
Long-term Short-term
Country averaging averaging Reference
mg/m3 ppm time(h) mg/m3 ppm time(min)
Bulgaria 0.008 0.006 24 0.008 0.006 30 Newill (1977)
China -- -- -- 0.01 0.007 20 Official
Communicationd
Czechoslovakia 0.008 0.006 24 0.008 0.006 30 Newill (1977)
German 0.008 0.006 24 0.015 0.011 10--30 Newill (1977)
Democratic
Republic
Federal
Germany, 0.005 0.004 24 0.01 0.007 30 Minister for
Federal Home Affairs
Republic of (1974)
Hungary 0.008a 0.006 24 0.008a 0.008 30 Newill (1977)
Hungary 0.15 0.11 24 0.3 0.21 30 Newill (1977)
Israel 0.045 0.032 24 0.15 0.11 30 Newill (1977)
Philippines -- -- -- 0.3 0.21 30 UNEP/IRPTCe
Poland 0.008b 0.006 24 0.0086 0.008 30 UNEP/IRPTCe
Poland 0.02c 0.014 24 0.06c 0.04 20 UNEP/IRPTCe
Romania 0.01 0.007 24 0.03 0.02 30 Newill (1977)
Spain 0.004 0.003 24 0.01 0.007 30 Newill (1977)
USSR 0.008 0.006 24 0.008 0.006 30 Newill (1977)
Yugoslavia 0.008 0.006 24 0.008 0.006 30 Newill (1977)
a For highly protected and protected areas.
b For especially protected areas, sanatoria, health resorts, sanctuaries, and
national parks.
c For protected areas, towns, and villages.
d Official communication from the Institute of Health, Chinese Academy of Medical
Sciences.
e Private communication.
Note by the WHO Task Group
Ambient Air Quality Standards have been set according to different
criteria in different countries, and these criteria may include, but
are not necessarily limited to the assessment of health effects.
Moreover, the limits themselves may have different meanings such as
the maximum acceptable level or the permissible level over a 10 to
30-min averaging time, etc.
Annex table 2. Occupational exposure standards for hydrogen sulfide
from selected countriesa
Country mg/m3 ppm Standard type
Australia 15 10 8-h TWAb
Belgium 15 10 8-h TWAb
Bulgaria 10 ceiling
China 10 ceiling
Czechoslovakia -- average 10 shiftc TWAb
maximum 20 10-min STELd
Finland 15 10 8-h TWAb
German Democratic Republic -- average 15 8.75-h TWA
short-term 15 30-min STELd
Germany, Federal Republic of 15 10 8-h TWAb
Hungary 10 8-h TWAb
Italy 10 shiftc TWAb
Japan 15 10 shiftc TWAb
Netherlands 15 10 shiftc TWAb
Poland 10 8-h TWAb
Romania -- average 10 shiftTWAb
maximum 15 ceiling
Sweden 15 10 shifte TWAb
Switzerland 15 10 8 to 9-h TWAb
USSR 10 less than 30-min STELd
USA -- occupational standard 20 ceiling except for one
10-min peak less than
50 ppm
ACGIH 15 10 8-h TWAb
21 15 15-min STELd
Yugoslavia 10 7 ceiling
a Abstracted from: ILO (1977).
b TWA = time-weighted average value.
c = a time-weighted value averaged over the entire shift or workday.
d STEL = short-term exposure limit.
No comments:
Post a Comment