Fire
Fire is the rapid oxidation of a material in the exothermic chemical process of combustion, releasing heat, light, and various reaction products. Slower oxidative processes like rusting or digestion are not included by this definition.
The flame is the visible portion of the fire. If hot enough, the gases may become ionized to produce plasma. Depending on the substances alight, and any impurities outside, the color of the flame and the fire's intensity will be different.
Fire in its most common form can result in conflagration, which has the potential to cause physical damage through burning. Fire is an important process that affects ecological systems across the globe. The positive effects of fire include stimulating growth and maintaining various ecological systems. Fire has been used by humans for cooking, generating heat, signaling, and propulsion purposes. The negative effects of fire include water contamination, soil erosion, atmospheric pollution and hazard to human and animal life.
Physical properties
Chemistry
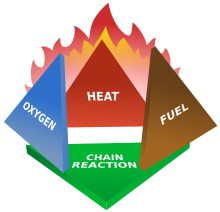
Fires start when a flammable and/or a combustible material, in combination with a sufficient quantity of an oxidizer such as oxygen gas or another oxygen-rich compound (though non-oxygen oxidizers exist that can replace oxygen), is exposed to a source of heat or ambient temperature above the flash point for the fuel/oxidizer mix, and is able to sustain a rate of rapid oxidation that produces a chain reaction. This is commonly called the fire tetrahedron. Fire cannot exist without all of these elements in place and in the right proportions. For example, a flammable liquid will start burning only if the fuel and oxygen are in the right proportions. Some fuel-oxygen mixes may require a catalyst, a substance that is not directly involved in any chemical reaction during combustion, but which enables the reactants to combust more readily.Once ignited, a chain reaction must take place whereby fires can sustain their own heat by the further release of heat energy in the process of combustion and may propagate, provided there is a continuous supply of an oxidizer and fuel.
Fire can be extinguished by removing any one of the elements of the fire tetrahedron. Consider a natural gas flame, such as from a stovetop burner. The fire can be extinguished by any of the following:
- turning off the gas supply, which removes the fuel source;
- covering the flame completely, which smothers the flame as the combustion both uses the available oxidizer (the oxygen in the air) and displaces it from the area around the flame with CO2;
- application of water, which removes heat from the fire faster than the fire can produce it (similarly, blowing hard on a flame will displace the heat of the currently burning gas from its fuel source, to the same end), or
- application of a retardant chemical such as Halon to the flame, which retards the chemical reaction itself until the rate of combustion is too slow to maintain the chain reaction.
Flame
The glow of a flame is complex. Black-body radiation is emitted from soot, gas, and fuel particles, though the soot particles are too small to behave like perfect blackbodies. There is also photon emission by de-excited atoms and molecules in the gases. Much of the radiation is emitted in the visible and infrared bands. The color depends on temperature for the black-body radiation, and on chemical makeup for the emission spectra. The dominant color in a flame changes with temperature. The photo of the forest fire is an excellent example of this variation. Near the ground, where most burning is occurring, the fire is white, the hottest color possible for organic material in general, or yellow. Above the yellow region, the color changes to orange, which is cooler, then red, which is cooler still. Above the red region, combustion no longer occurs, and the uncombusted carbon particles are visible as black smoke.
The National Aeronautics and Space Administration (NASA) of the United States has recently found that gravity also plays a role in flame formation. Modifying the gravity causes different flame types The common distribution of a flame under normal gravity conditions depends on convection, as soot tends to rise to the top of a general flame, as in a candle in normal gravity conditions, making it yellow. In micro gravity or zero gravity, such as an environment in outer space, convection no longer occurs, and the flame becomes spherical, with a tendency to become more blue and more efficient (although it may go out if not moved steadily, as the CO2 from combustion does not disperse as readily in micro gravity, and tends to smother the flame). There are several possible explanations for this difference, of which the most likely is that the temperature is sufficiently evenly distributed that soot is not formed and complete combustion occurs.Experiments by NASA reveal that diffusion flames in micro gravity allow more soot to be completely oxidized after they are produced than diffusion flames on Earth, because of a series of mechanisms that behave differently in micro gravity when compared to normal gravity conditions. These discoveries have potential applications in applied science and industry, especially concerning fuel efficiency.
In combustion engines, various steps are taken to eliminate a flame. The method depends mainly on whether the fuel is oil, wood, or a high-energy fuel such as jet fuel.
Heat
Fires give off heat, or the process of energy transfer from one body or system due to thermal contact.Typical temperatures of fires and flames
- Oxyhydrogen flame: 2000 °C or above (3600 °F.
- Bunsen burner flame: 1,300 to 1,600 °C (2,400 to 2,900 °F).
- Blowtorch flame: 1,300 °C (2,400 °F).
- Candle flame: 1,000 °C (1,800 °F)
- Smoldering cigarette:
- Temperature without drawing: side of the lit portion; 400 °C (750 °F); middle of the lit portion: 585 °C (1,100 °F)
- Temperature during drawing: middle of the lit portion: 700 °C (1,300 °F)
- Always hotter in the middle.
Temperatures of flames by appearance
The temperature of flames with carbon particles emitting light can be assessed by their color:- Red
- Just visible: 525 °C (980 °F)
- Dull: 700 °C (1,300 °F)
- Cherry, dull: 800 °C (1,500 °F)
- Cherry, full: 900 °C (1,700 °F)
- Cherry, clear: 1,000 °C (1,800 °F)
- Orange
- Deep: 1,100 °C (2,000 °F)
- Clear: 1,200 °C (2,200 °F)
- White
- Whitish: 1,300 °C (2,400 °F)
- Bright: 1,400 °C (2,600 °F)
- Dazzling: 1,500 °C (2,700 °F)





No comments:
Post a Comment