Total organic carbon (TOC) is the amount of carbon found in an organic
compound and is often used as a non-specific indicator of water quality
or cleanliness of pharmaceutical manufacturing equipment. TOC may also
refer to the amount of organic carbon in soil, or in a geological
formation, particularly the source rock for a petroleum play; 2% is a
rough minimum.For marine surface sediments, average TOC content is 0.5%
in the deep ocean, and 2% along the eastern margins.
understand the analysis process better, some key basic terminologies should be understood and their relationships to one another
Total Carbon (TC) – all the carbon in the sample, including both inorganic and organic carbon
Total Inorganic Carbon (TIC) – often referred to as inorganic carbon (IC), carbonate, bicarbonate, and dissolved carbon dioxide (CO2).
Total Organic Carbon (TOC) – material derived from decaying vegetation, bacterial growth, and metabolic activities of living organisms or chemicals.
Elemental Carbon (EC) – charcoal, coal, and soot. Resistant to analytical digestion and extraction, EC can be a fraction of either TIC or TOC depending on analytical approach.[8]
Non-Purgeable Organic Carbon (NPOC) – commonly referred to as TOC; organic carbon remaining in an acidified sample after purging the sample with gas.
Purgeable (volatile) Organic Compound (VOC) – organic carbon that has been removed from a neutral, or acidified sample by purging with an inert gas. These are the same compounds referred to as Volatile Organic Compounds (VOC) and usually determined by Purge and Trap Gas Chromatography.
Dissolved Organic Carbon (DOC) – organic carbon remaining in a sample after filtering the sample, typically using a 0.45 micrometer filter.
Suspended Organic Carbon – also called particulate organic carbon (POC); the carbon in particulate form that is too large to pass through a filter.Total organic carbon (TOC) is the amount of carbon found in an organic compound and is often used as a non-specific indicator of water quality or cleanliness of pharmaceutical manufacturing equipment. TOC may also refer to the amount of organic carbon in soil, or in a geological formation, particularly the source rock for a petroleum play; 2% is a rough minimum.For marine surface sediments, average TOC content is 0.5% in the deep ocean, and 2% along the eastern margins.
understand the analysis process better, some key basic terminologies should be understood and their relationships to one another
Total Carbon (TC) – all the carbon in the sample, including both inorganic and organic carbon
Total Inorganic Carbon (TIC) – often referred to as inorganic carbon (IC), carbonate, bicarbonate, and dissolved carbon dioxide (CO2).
Total Organic Carbon (TOC) – material derived from decaying vegetation, bacterial growth, and metabolic activities of living organisms or chemicals.
Elemental Carbon (EC) – charcoal, coal, and soot. Resistant to analytical digestion and extraction, EC can be a fraction of either TIC or TOC depending on analytical approach.[8]
Non-Purgeable Organic Carbon (NPOC) – commonly referred to as TOC; organic carbon remaining in an acidified sample after purging the sample with gas.
Purgeable (volatile) Organic Compound (VOC) – organic carbon that has been removed from a neutral, or acidified sample by purging with an inert gas. These are the same compounds referred to as Volatile Organic Compounds (VOC) and usually determined by Purge and Trap Gas Chromatography.
Dissolved Organic Carbon (DOC) – organic carbon remaining in a sample after filtering the sample, typically using a 0.45 micrometer filter.
Suspended Organic Carbon – also called particulate organic carbon (POC); the carbon in particulate form that is too large to pass through a filter.
Carbon content is one of the most important parameters measured in
various types of solutions, from commercial drinking water to industrial
wastewater. Total organic carbon1 (TOC) analyzers are
devices used to analyze the organic carbon content in these water or
liquid solutions. They provide highly sensitive, non-specific readouts
of all TOC through two-stage processes involving oxidation and
detection. These devices are necessary to account for chemical solvents
and bacteria that contaminate water solutions. This is important because
of the harmful effects that TOCs may have on health and the
environment.
Table 1 – Examples of water sample types and their average TOC levels. Table Credit: Tekmar-Dohrmann
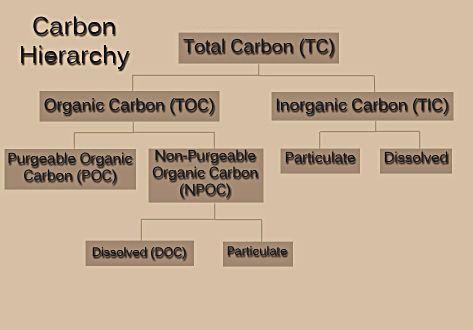
In analyzing a material that contains carbon, one must first understand the different types of carbon that may be present. Figure 1 shows a hierarchy of carbon types.

Figure 1 – Breakdown of carbon types.
In order to analyze a sample, TOC analyzers employ either differential or direct methods of measurement.
In the differential method (also known as TC-IC), both total carbon (TC) and total inorganic carbon2 (TIC) may be determined by separate measurement and TOC may be calculated by subtracting TIC from TC. This method is suitable for samples in which TIC is less than or of similar size to TOC.
In the direct method, TIC is removed from a sample by purging3 the acidified sample with a purified gas. TOC is then determined by oxidation. This method is also known as NPOC (non-purgeable organic carbon) due to the fact that POC (purgeable organic carbon) such as benzene, toluene, cyclohexane, and chloroform may be partly removed from a sample by gas stripping. The direct method is suitable for surface water, ground water, and drinking water because of (in most cases) less TOC than IC and a negligible amount of POC in these samples.
Table 2 – Overview of different types of analyzers based on oxidation and detection methods. Table Credit: Tekmar-Dohrmann
Table 3 – TOC analysis method for different water samples. Table Credit: Tekmar-Dohrmann
Non-dispersive infrared (NDIR) cell detection
measures carbon dioxide in the gas phase. It is a direct method that
specifically measures carbon dioxide by analyzing the absorption
spectrum in the IR region. Static pressurized concentration (SPC) is a
new NDIR technology. In this method, the detector is pressurized. Once
the gases contained in the detector reach equilibrium, carbon dioxide
concentration is analyzed. A TOC analyzer that utilizes this process measures all of the oxidation products contained in the sample in a single reading.
understand the analysis process better, some key basic terminologies should be understood and their relationships to one another
Total Carbon (TC) – all the carbon in the sample, including both inorganic and organic carbon
Total Inorganic Carbon (TIC) – often referred to as inorganic carbon (IC), carbonate, bicarbonate, and dissolved carbon dioxide (CO2).
Total Organic Carbon (TOC) – material derived from decaying vegetation, bacterial growth, and metabolic activities of living organisms or chemicals.
Elemental Carbon (EC) – charcoal, coal, and soot. Resistant to analytical digestion and extraction, EC can be a fraction of either TIC or TOC depending on analytical approach.[8]
Non-Purgeable Organic Carbon (NPOC) – commonly referred to as TOC; organic carbon remaining in an acidified sample after purging the sample with gas.
Purgeable (volatile) Organic Compound (VOC) – organic carbon that has been removed from a neutral, or acidified sample by purging with an inert gas. These are the same compounds referred to as Volatile Organic Compounds (VOC) and usually determined by Purge and Trap Gas Chromatography.
Dissolved Organic Carbon (DOC) – organic carbon remaining in a sample after filtering the sample, typically using a 0.45 micrometer filter.
Suspended Organic Carbon – also called particulate organic carbon (POC); the carbon in particulate form that is too large to pass through a filter.Total organic carbon (TOC) is the amount of carbon found in an organic compound and is often used as a non-specific indicator of water quality or cleanliness of pharmaceutical manufacturing equipment. TOC may also refer to the amount of organic carbon in soil, or in a geological formation, particularly the source rock for a petroleum play; 2% is a rough minimum.For marine surface sediments, average TOC content is 0.5% in the deep ocean, and 2% along the eastern margins.
understand the analysis process better, some key basic terminologies should be understood and their relationships to one another
Total Carbon (TC) – all the carbon in the sample, including both inorganic and organic carbon
Total Inorganic Carbon (TIC) – often referred to as inorganic carbon (IC), carbonate, bicarbonate, and dissolved carbon dioxide (CO2).
Total Organic Carbon (TOC) – material derived from decaying vegetation, bacterial growth, and metabolic activities of living organisms or chemicals.
Elemental Carbon (EC) – charcoal, coal, and soot. Resistant to analytical digestion and extraction, EC can be a fraction of either TIC or TOC depending on analytical approach.[8]
Non-Purgeable Organic Carbon (NPOC) – commonly referred to as TOC; organic carbon remaining in an acidified sample after purging the sample with gas.
Purgeable (volatile) Organic Compound (VOC) – organic carbon that has been removed from a neutral, or acidified sample by purging with an inert gas. These are the same compounds referred to as Volatile Organic Compounds (VOC) and usually determined by Purge and Trap Gas Chromatography.
Dissolved Organic Carbon (DOC) – organic carbon remaining in a sample after filtering the sample, typically using a 0.45 micrometer filter.
Suspended Organic Carbon – also called particulate organic carbon (POC); the carbon in particulate form that is too large to pass through a filter.

Total Organic Carbon (TOC) Analyzers Information
|

|

|
|
Type of Water
|
TOC (mg/L)
|
|
High purity water
|
<0.01
|
|
Water for injection
|
<0.50
|
|
Ground water
|
<1
|
|
Seawater
|
<1
|
|
Drinking water
|
<4
|
|
Surface water
|
<10
|
|
Wastewater
|
>10
|
In analyzing a material that contains carbon, one must first understand the different types of carbon that may be present. Figure 1 shows a hierarchy of carbon types.

Figure 1 – Breakdown of carbon types.
In the differential method (also known as TC-IC), both total carbon (TC) and total inorganic carbon2 (TIC) may be determined by separate measurement and TOC may be calculated by subtracting TIC from TC. This method is suitable for samples in which TIC is less than or of similar size to TOC.
In the direct method, TIC is removed from a sample by purging3 the acidified sample with a purified gas. TOC is then determined by oxidation. This method is also known as NPOC (non-purgeable organic carbon) due to the fact that POC (purgeable organic carbon) such as benzene, toluene, cyclohexane, and chloroform may be partly removed from a sample by gas stripping. The direct method is suitable for surface water, ground water, and drinking water because of (in most cases) less TOC than IC and a negligible amount of POC in these samples.
Selection
When selecting a TOC analyzer, industrial buyers should focus on the analysis methods and performance specifications of the device.Methods of Analysis
TOC analyzers can employ a number of different techniques for oxidation and detection of TOC. These methods generally have a specific analytical range and many have been officially standardized. The table below provides an overview of this information.|
Oxidation
|
Detection Technique
|
Analytical Range
|
Official Methods
|
|
UV/persulfate4
|
NDIR5
|
0.002 to 10,000 mg/L
|
EPA 415.1, 9060A Standard Methods 5310C ASTM D2579, ISO (Draft) 8245, AOAC 973.47, USP 643
|
|
Heated persulfate
|
NDIR
|
0.002 to 1,000 mg/L
|
EPA 415.1, 9060A Standard Methods 5310C ASTM D2579, ISO (Draft) 8245, AOAC 973.47, USP 643
|
|
Combustion
|
NDIR
|
0.004 to 25,000 mg/L
|
EPA 415.1, 9060A Standard Methods 5310B ASTM D2579, ISO (Draft) 8245, AOAC 973.47, USP 643
|
|
UV/persulfate
|
Membrane/conductivity
|
0.0005 to 50 mg/L
|
Standard Methods 5310C, USP 643
|
|
UV
|
Conductivity or NDIR
|
0.0005 to 0.5 mg/L
|
USP 643
|
Oxidation
All TOC analyzers offered today employ either combustion or low-temperature oxidation techniques. The purpose of the oxidation step is to convert the organics to carbon dioxide which can be measured by a detector to provide TOC values.- Low-temperature oxidation is chemical oxidation
aided by 100°C heat and either persulfate, UV irradiation and
persulfate, or only UV irradiation. The low-temperature techniques have
the advantage of allowing a large volume of sample to be analyzed,
thereby improving the low limit of detection. Also, the blank value is
very low as long as the reagents are pure, which makes the analysis more
accurate.
- Ultraviolet irradiation uses ultra-violet light to oxidize the carbon present in the sample to produce carbon dioxide. UV oxidation is the most reliable, low maintenance method for TOC analysis of ultra-pure waters.
- Thermo-chemical (persulfate) oxidation, also called “heated persulfate”, uses the same free radical formation method as UV/persulfate oxidation, but also uses heat to magnify the oxidation power of persulfate. Unlike UV/persulfate, this method is not susceptible to lower recoveries resulting from turbidity in samples. TOC analyzers which incorporate thermo-chemical oxidation are useful in wastewater, drinking water, and pharmaceutical water analysis. When used with sensitive non-dispersive infrared (NDIR) cell detectors, these analyzers can readily measure TOC up to hundreds of ppm depending on samples volumes.
- UV/persulfate irradiation uses UV light for oxidation magnified by a chemical oxidizer (usually persulfate). TOC analyzers that use this oxidation method can measure levels of organic carbon as low as 200 parts per billion (ppb).
- High temperature combustion uses heat (680°C or higher) in a stream of air or pure oxygen and usually in the presence of a catalyst. The catalysts used are generally cupric oxide, cobalt oxide, or platinum on an alumina support. In the process, the oxygen-rich atmosphere converts all carbon to carbon dioxide to be measured. Most modern analyzers use NDIR to detect the carbon dioxide produced from combustion.
|
Application
|
TOC Analysis Method
|
Comments
|
|
High purity water
|
UV or UV/persulfate
|
Samples
with less than 50 ppb TOC. UV or UV/persulfate provides the most
trouble free operation and the ease of detecting very low level TOC.
|
|
Water for injection
|
UV or UV/persulfate
|
Samples
with less than 500 ppb TOC. Combustion technology can measure in this
range, but UV or UV/persulfate is still the easier method.
|
|
Drinking or source water
|
UV/persulfate or combustion
|
For
meeting precision and accuracy requirements, UV/persulfate is the
better technology. Combustion should be used if capturing the
particulated organic carbon as part of the TOC value is important.
|
|
Salty water
|
Combustion
|
Combustion technology is not affected by chlorides present in seawater and other salty matrixes.
|
|
Industrial waste effluent
|
Combustion
|
Combustion
technology was designed to handle particulates, chlorides, and tough to
oxidize compounds typical of industrial waste.
|
Detection
Out of all components of a TOC analyzer, accurate detection and quantification are considered the most vital. Detection techniques include conductivity cells and non-dispersive infrared cells.- Conductivity cells rely on aqueous CO2
to raise acidity levels, causing the conductivity of the solution to
increase. TOC analyzers that use a conductometric method can be used for
the measurement of carbon dioxide in the liquid phase. Unlike NDIR
detection, this method displays an extremely stable calibration and is
not susceptible to considerable drift over time. This means that the TOC
analyzer can be calibrated less frequently without compromising on
analytical performance. Direct conductivity and membrane conductivity
are the two types of conductivity detectors.
- Direct conductivity detectors provide simple and inexpensive CO2 measurement, using no carrier gas. They suffer from an analytical range limited to parts per billion measurement, and are also susceptible to interference from ionic contamination, halogenated organic material, acids, and bases.
- Membrane conductivity detectors are more robust than direct devices, with a wide analytical range for both deionized and ionized water samples. In membrane conductivity, the membrane forms a protective barrier to interfering ions, enabling the exclusive analysis of carbon dioxide. This method suffers from a slow analysis time and is also prone to ‘false negatives’ due to the membrane acting as a site for secondary chemical reactions.
Performance Specifications
One of the most important specifications provided for total organic carbon (TOC) analyzers is the measuring range. Accuracy, resolution, and analysis time are important as well.- Analytical range is the range of TOC values that a device can analyze effectively. Analyzers with a long range can measure a wider variety of sample types. Range can be specified in mg/L (milligrams carbon per liter solution) or on a parts per million/billion basis.
- Accuracy determines how much a device’s TOC value may deviate from the actual value.
- Resolution is the detail of an analyzer’s TOC measurement. A high resolution device may read in the parts per billion range, while a lower resolution device may only measure TOC up to parts per million.
- Analysis time is the time the device takes to complete a sample analysis. Times may range from minutes to hours depending on the methods used by the technology.
Definition of Terms
- Organic carbon – Carbon within an organic compound. Organic compounds are (generally) those of biological origin.
- Inorganic carbon – Carbon within an inorganic compound. Inorganic compounds are (generally) those of inanimate or non-biological origin.
- Purging – Removal of gases in a solution or contained vessel by filling and rinsing with an inert gas such as gaseous nitrogen (GAN).
- Persulfates – Ions or compounds with an excess of oxygen, such as permonosulfate (SO52-).
- NDIR or nondispersive infrared sensor – Spectroscopic device used as a gas detector. Composed of an infrared light, sample chamber, filter, and infrared detector.
No comments:
Post a Comment