Copper (Cu++)
Function
Copper is essential for many plant functions. Some of them are
- It functions as a catalyst in photosynthesis and respiration.
- It is a constituent of several enzyme systems involved in building and converting amino acids to proteins.
- Copper is important in carbohydrate and protein metabolism.
- It is important to the formation of lignin in plant cell walls which contributes to the structural strength of the cells, and the plant.
- Copper also affects the flavor, the storageability, and the sugar content of fruits.
Factors Affecting Availability
- Root Growth: Copper is the most immobile micronutrient, therefore anything that inhibits new root growth will inhibit Cu uptake.
- Soil pH: Acid soils increase Cu uptake and High pH inhibits uptake.
- Organic Matter: Copper is readily and tightly complexed by organic matter, therefore high soil organic matter levels reduce Cu availability.
- Flooding: Waterlogged soils can reduce Cu availability while they are saturated, however after they are drained the Cu will become available again.
- Cu:Zn Balance: High Zn levels will reduce Cu availability.
- Cu:N Balance: High N uptake in the presence of marginal Cu levels can lead to a reduction of Cu transport into the growing tips of plants.
- Cu:P Balance: High soil and plant P levels can reduce Cu uptake due to reduced soil exploration by mycorrhizas associated with plant roots.
- N Stress: Low N availability decreases the vigor of plants to an extent that it may fail to take up adequate amounts of many other nutrients. Copper uptake can be affected in this way.
High Response Crops
While this is an essential element for all plants, these crops have been found to be especially responsive: alfalfa, barley, blueberry, beet, broccoli, carrot, cabbage, celery, eggplant, flax, lettuce, oats, onion, parsnip, pepper, rye, spinach, sudangrrass, tomato, watermelon, and wheat.
Deficiency Symptoms
Young tissues show chlorosis, distortion, and necrosis (death). The death of the growing points often leads to excessive tillering in cereal crops and excessive branching in dicots (non-grass crops). Some vegetables show a blue-green color before advancing to chlorosis. Excessive wilting, lodging and reduced disease resistance result from the weak cell walls caused by Cu deficiency. Reduced seed and fruit yield is caused mainly by male sterility. Copper deficiency often causes a complete failure to set flowers. Lettuce and onions most commonly manifest visible symptoms with only a slight deficiency occurring. Copper is found to be evenly distributed in the plant, but is relatively immobile. Therefore, a constant supply is needed throughout the growing season.
Toxicity
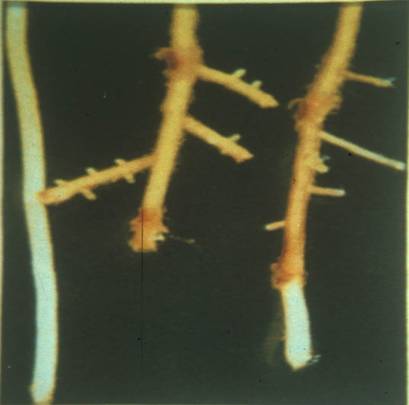
Copper should not be applied to soils without a demonstrated need through soil and plant analysis. Toxic effects from over-application can last many years. Symptoms appear in young tissue and include; dark green leaves followed by induced Fe chlorosis in which the leaves may appear nearly white; thick, short, or barbed-wire looking roots which can be mistaken for chemical damage; depressed tillering. Work has shown that soils high in iron (Fe), or plants that are exceptionally efficient in absorbing Fe can increase the plant concentration at which Cu has toxic effects, thus lessening the toxic effect. Increasing the soil pH should also help reduce toxic effects, although this can cause deficiencies of other nutrients. In situations where toxic soil levels of Cu exist, the leaf analysis may not properly reflect the severity of the problem because the root damage can become self-limiting to Cu uptake. If the field has a history of having been an arbor, orchard, or of any crop where “Bordeaux Mix” may have been used for many years, you should suspect excess soil Copper. Liming to the proper pH, using Molybdenum seed treatments, and increasing the Nitrogen, Zinc and Phosphorus rates will help minimize the effects of High soil Copper. Although this is not well proven, foliar Fe might help offset the damaging effects of excess Cu.
Using Copper in a Fertilizer Program
Soil testing is the first step in determining a need. Plant analyses are also useful, and when a need is determined treatment should follow.
| Recommended rates of Cu | |
|---|---|
| Method | Rate |
| Broadcast: | 1.0 to 10.0 lb./A |
| In-row (2×2): | 1.0 to 5.00 lb./A |
| Foliar: | 0.1 to 0.25 lb./A |
Correcting problems is usually not difficult. Again proper liming must be considered as a basic step. Remember, excess Cu applications can easily damage plant roots and leaves, so proper application rates and methods are important. Soil applications of Copper materials can have an extremely long residual effect in the soil. Therefore, records must be kept on the total amounts applied to fields. If a foliar Cu product is “basic” in nature (the pH of the Copper product/carrier mixture is greater than 7.0), the potential for, and severity of foliar damage can be reduced. Good responses have been obtained from foliar applications of Copper-containing fungicides.
| Some Copper containing fertilizer materials | ||
|---|---|---|
| Product | Chemical Formula | Typical Cu % |
| Copper Sulfate Monohydrate | CuSO4iH2O | 35% |
| Copper Sulfate Pentahydrate | CuSO4i5H2O | 25% |
| Cupric Oxide | CuO | 75% |
| Copper Chloride | CuCl2 | 17% |
| Copper Chelates | CuEDTA | 8-13% |

No comments:
Post a Comment