Phosphorus (P)
Functions of Phosphorus
Phosphorus is essential to many plant functions and structures. It plays a role in
- Photosynthesis
- Respiration
- Seed and fruit production
- Energy production, storage, and transfer
- Cell division and enlargement
Adequate supplies of P promote or enhance
- Early root formation and growth
- Greater flowering and seed production
- Fruit, vegetable, and grain quality
- Better growth in cold temperatures
- Water use efficiency
- Early maturation of fruit and grain
The primary functions of P in plants are
- Structural component of proteins, enzymes, nucleic acids, and DNA
- Photosynthesis (production of sugars and starches)
- Respiration (producing energy by oxidizing sugars and starches)
Phosphorus compounds form part of the structure of amino acids, proteins, nucleic acids, and DNA (Fig. 1). It is obvious that without DNA plants cannot reproduce, which means that they cannot produce the seed and fruit that we harvest from many crops. Without nucleic acids plant cells cannot develop or function properly.
Less obvious are the many different roles that various proteins play in the proper functioning of plants. For example, some proteins are essential for the formation and proper function of enzymes, which are involved in many plant processes, including photosynthesis.
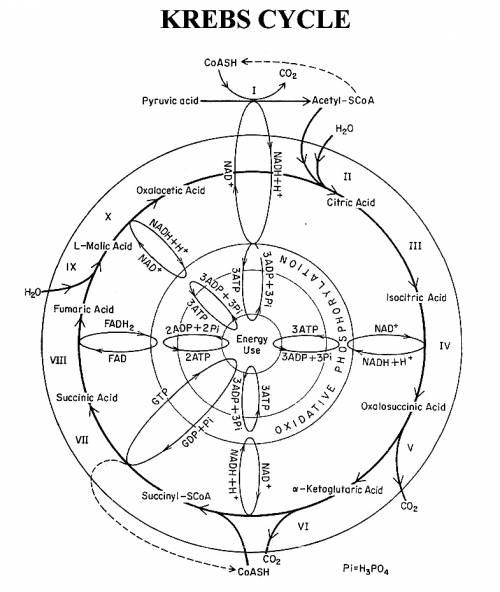
Phosphorus plays a central role in both photosynthesis and respiration. Both are exceptionally complex processes, as illustrated by the Krebs cycle (Fig. 2). The Krebs cycle is the second step in respiration, the process within which plants produce the energy needed to exist and grow. Compounds containing P such as NAD, NADH, ADP, and ATP are key components of the Krebs cycle.
The production of sugars during photosynthesis and the conversion of these sugars into energy during respiration enable the plant to perform all other life-functions. When respiration is restricted due to a P shortage, sugars are not converted into energy and they accumulate within the plant tissue. The accumulation of unused sugars leads to the purple coloration often seen with P deficiency. The low energy level within the plants is the underlying cause of the stunted growth typically seen with P deficiency. When energy is low, all plant processes suffer. Flowering and reproduction place a high demand for energy on plants (not to mention the need for DNA in seed production after fertilization). Therefore, adequate P is essential to the process. A plants ability to generate abundant energy becomes more important when it is put under additional stress, such as cold soil and air temperatures.
Crops Utilize Large Amounts of Phosphorus
| Phosphorus Uptake | ||||
|---|---|---|---|---|
| Crop | Yield | Harvested Portion | Residue | Total Uptake |
| lb/ac | lb/ac | lb/ac | ||
| Alfalfa | 8 Ton | 120 | 0 | 120 |
| Coastal Bermuda | 8 ton | 112 | 0 | 112 |
| Corn (grain) | 150 Bu. | 66 | 37 | 103 |
| Corn (silage) | 20 Ton | 72 | 0 | 72 |
| Cotton (lint) | 3 bales | 36 | 27 | 63 |
| Fescue | 5 ton | 93 | 0 | 93 |
| Peppers | 14 Ton | 20 | 13 | 33 |
| Potatoes | 25 Ton | 69 | 27 | 96 |
| Rice | 7,000 lb. | 42 | 8 | 50 |
| Sorghum (grain) | 6,000 lb. | 45 | 17 | 62 |
| Soybeans | 60 Bu. | 48 | 18 | 66 |
| Tomatoes | 50 ton | 65 | 26 | 91 |
| Wheat | 80 Bu. | 44 | 15 | 59 |
Phosphorus in the Soil
Phosphorus is a highly reactive element, and as such, does not exist in the elemental form in the soil. The majority of P in most soil is in essentially insoluble forms, and unavailable to plants. In fertile soil a significant portion of the total P is in moderately soluble forms, which act as a “ready reserve” to replenish the pool of soluble P as it is depleted by plant growth and other organisms.
Plant roots take up nearly all P as either the primary or secondary orthophosphate anion (H2PO4- or HPO4-2, respectively). Primary orthophosphate is the form that is dominant in acid soils and is taken up about 10 times as readily as the secondary orthophosphate form. At a soil pH of 7.0 there is approximately equal amounts of the two P forms and as the soil pH increases above pH 7.0, the secondary orthophosphate ion becomes the dominant form of available P.
All P sources applied to the soil must be converted to the orthophosphate forms before a plant can utilize them. However, applying these forms of P to the soil does not guarantee that they will remain in that form for very long. Because phosphorus is highly reactive, it is readily converted to other, less soluble forms. The particular forms that are created depend on other soil factors such as the soil pH, temperature, moisture, other elements, and others. This is one reason all aspects of the soil must be optimized before plants will perform at their best.
Because phosphorus is essentially immobile in most soils, any factor that reduces normal root growth and function, for example soil compaction, will likely result in a P deficiency in those plants. This occurs even on soils with adequate to high P tests.
In most situations there is very little soluble P in the soil at any point in time. It has been estimated that at any point in time, the solution/available forms of P in many soils may only amount to from 0.01 to 0.06 ppm (0.02-0.12 lb P/acre). This P will typically move no more than about one tenth of an inch in the soil. Roots quickly deplete the 0.10 inch cylinder of soil around each root and must continually grow into new areas of the soil to maintain adequate P intake.
The fertilizer form of P is referred to as P205 (phosphorous pentoxide), however, no such compound actually exists in fertilizer or the soil. This is simply a uniform way of equating the various forms of P to each other. To convert P to P205 multiply P × 2.3. To convert P205 to P, either divide P205 by 2.3 or multiply P205 × 0.435.
Factors Affecting P Availability
At the risk of stating the obvious, higher soil P tests are typically the most significant factor controlling P availability to crops. However, P availability can be strongly affected by several other soil factors
Soil pH: As the soil pH increases above about pH 7.0, soil P is increasingly “fixed” into less soluble/available forms by excess calcium. As the soil pH decreases below about pH 6.0, soil P is increasingly “fixed” into less soluble/available forms by excess soluble aluminum.
Soil Compaction: Phosphorus moves very little in the soil. Because of this, plant roots must be healthy and actively explore new areas of the soil daily in order to obtain adequate P nutrition. Anything that inhibits aggressive root growth is likely to reduce P uptake, even in high P soils.
Soil Aeration: Inadequate soil aeration is often related to soil clay content, soil drainage, and soil compaction. Most cultivated plants require adequate oxygen (O2) in the soil atmosphere. A lack of adequate soil O2 can reduce P uptake by as much as 50%.
Soil Moisture: As moisture stress increases, P availability and uptake decrease. Higher levels of soil P result in higher P uptake at all moisture levels. However, as soil moisture begins to exceed field capacity, the excess water excludes the needed oxygen from the soil and P uptake begins to suffer due to the lack of O2 in the soil.
Soil Temperature: Cold soil reduces P uptake, as well as most other chemical and biological activity in the soil. This is the reason that many fields respond to row-placed fertilizer. As the summer begins to warm up, uptake efficiency of both the plants and the soil improve. However, permanent yield losses can occur from early season P shortages.
Soil Texture: Generally, low CEC soils require higher soil P tests to supply equivalent amounts of P to a crop. Such soils typically hold less water at any point in time, which slows P diffusion to the roots. These soils also have less particle surface area and it appears that current soil testing procedures may extract a higher percent of the total P in lower CEC soils. This would lead to less capacity to quickly replenish the P in solution (buffering power) and require a proportionately higher soil P level for equivalent P supplying power. Some clay types have a high P fixation capacity. These types of clay are more common in tropical soils. In these cases, we would logically expect a higher CEC to require a proportionately higher soil P level for adequate soil fertility.
Soil Organic Matter: The organic matter (OM) in soil may account for anywhere from 3% to 75% of the total P in a soil (not necessarily the same as “available P”). Generally, increased OM results in greater fixation of Fe and Al, resulting in less P fixation by these elements, and more labile (available) P. Such reactions also tend to reduce the fixation of applied P as well. Typically, in soils developed in temperate climates, the contribution of P by OM is relatively small and the main source of P for plants is the inorganic forms.
Crop Residues: Incorporation of large amounts of crop residues can result in immobilization of available P by microbes. As they decompose the residue, they grow and reproduce, thus creating their own need for available P. During the decomposition of crop residue, soil microbes are effectively in competition with higher plants. Microbially immobilized P will gradually become available as decomposition is completed, the microbes die, and they are re-cycled. Factors such as temperature, moisture, soil pH, soil aeration, and the availability of other nutrients have a direct bearing on the level of microbial activity in the soil and the rates of immobilization and mineralization. While this cycle is present in all soils, it is not thought to be a cause for major adjustments in fertilizer recommendation programs.
Plant Root Systems: As mentioned earlier, soil P is essentially immobile, and the portion of soil P that is soluble and immediately available to plants is exceptionally small. Therefore, plant roots must constantly explore large volumes of soil to satisfy their need for P. There are significant differences between species in the relative size and effectiveness of their root systems. There can also be significant differences of this type between hybrids and varieties within the same species. This is one factor in explaining why some plants or crops require different soil P levels for a given level of performance.
Mycorrhizae: Mycorrhizae are soil fungi that form a symbiotic association with plant roots. The thread-like hyphae of the fungus connect with plant roots and extend into the soil. The hyphae act like extensions of the plants root system by absorbing nutrients and transporting them back to the plant roots. A major benefit in this respect is an increase in P uptake. In exchange, the mycorrhizae receive sugars manufactured by the plant.
While mycorrhizae can infect most plants, they typically are more of a benefit to trees than agricultural crops. It has been demonstrated with agricultural crops that the benefits of mycorrhizae decrease as the soil P level increases. Soil P levels adequate for good yields of most crops essentially eliminate the benefits of Mycorrhizae.
In flooded soils, mycorrhizae may die. If the soil has a low level of available P, the loss of the Mycorrhizae may greatly decrease the following crops ability to absorb enough P. This effect has been seen in wheat following flooded rice crops.
Interactions of P with Other Elements
Nitrogen: Many observations have found that P uptake is enhanced when in combination with ammonium N (NH4-N). In most cases, NH4-N has been shown to be superior to other forms of N at enhancing P uptake. This benefit typically requires that the N and P be applied in either a chemically combined form or as a concentrated mixture, such as a banded fertilizer blend. The exact mechanism for this reaction is not clearly understood. However, it is thought that as the NH4-N undergoes nitrification, P uptake is increased. It is also well known that increased N uptake stimulates the uptake of many other elements, and this may play a role in the effect.
Potassium: Potassium has been shown to co-precipitate with P when soluble phosphoric fertilizers are applied to soils. This effect is more pronounced in soils with high exchangeable K levels or with easily decomposed K-bearing minerals. However, this reaction has rarely been demonstrated to have a significant effect on plant growth. There is little or no evidence to show an interaction between P and K within the plant.
Calcium: As mentioned in the section on pH, calcium will combine with P to make insoluble compounds that are unavailable to plants in the short term. The general trend in the reaction is that as the soil Ca content and pH increase more P will combine with Ca to form compounds with ever-decreasing solubility. In these situations, it is typical to find that crops will require a correspondingly higher soil P test for equal growth. Alternatively, growers have seen that banding P fertilizers, especially when the band can be made acidic, improves crop growth in these conditions.
Magnesium: Phosphorus and Mg are often highly reactive in fertilizer manufacturing processes. The result of the reaction being the formation of highly insoluble compounds that coat or clog equipment. However, this effect has not been demonstrated to be a concern in the soil. In fact, much work has shown that Mg fertilization can enhance P uptake by plants. Within plants, Mg is an activator of certain enzymes that are critical to P transfer and as such, proper Mg nutrition would be essential to the uptake and utilization of P within the plant.
Sulfate: There has been some work that suggests that sulfates (SO4-S) may compete with soluble phosphates (H2PO4-) for the limited amount of anion retention sites in soil. These retention sites appear to primarily be Al and Fe hydroxides. The effect of such a relationship would be that high applications of either element should displace the other. In theory, this would cause a short-term increase in the amount of the displaced element in the soil solution, possibly followed by increased leaching of that element. The long term effect could be a depletion of the displaced element. While it does not seem likely that high rates of applied SO4-S would have a significant effect on P movement in the soil, the reverse seems possible in some sandy soil.
Zinc: Phosphorus interactions have been studied and widely publicized for many years. The results have shown that high levels of either element can depress the uptake of the other. While we know that the interaction can occur, we do not know enough to accurately predict when problem will occur. However, when soil P tests are above about 100 to 150 lb. /acre by either the Bray-P1 or Mehlich 3 procedures, the possibility of depressed Zn uptake should be a concern. The problem may be more severe, or occur at a lower soil P test in soils with a pH significantly higher than 7.0. It is rare in everyday situations for Zn applications to reduce P uptake. However, it can occur under the right conditions.
Copper: High soil P levels can depress Cu uptake, especially when other Cu limiting conditions are present, such as high soil pH and high soil organic matter. As early as the 1940's it was found that high P applications alleviated Cu toxicity by reducing the availability of soil Cu in Florida citrus groves. Other work with citrus confirmed the original findings, but little work has been done with other crops. However, at Spectrum Analytic we see evidence of this effect in plant analysis samples each year. While our observations are not research, it is common to receive plant samples of various species where elevated P uptake occurs with low plant Cu levels. These situations often occur on soils where a Cu shortage would not otherwise be predicted.
Boron: There has been little research into the possible interactions between B and P. However, boron is an anion in the available form. As such, it reacts with Al and Fe oxides. Since this process is similar to that of soluble P, it seems reasonable that it may interact with P in much the same way as SO4-S. In the late 50's and early 60's, researchers in California reported that applications of Ca(H2PO4)2 resulted in lower availability of B, especially in acid soils.
Molybdenum: While the interaction between Mo and P has not been studied extensively, some rather convincing evidence indicates that P increases the short term uptake of Mo. In its available form, Mo is an anion. It is presumed that the reason for P increasing the uptake of Mo is similar to the same relationship of P with B and SO4-S. In all of these cases, both elements react with Al and Fe oxides. A comprehensive report by Stout et al (1951), found that P applications without SO4-S increased Mo uptake up to 10-fold. The beneficial effects of P on Mo uptake appeared to be stronger in acid soils. That study found that the same P applications plus SO4-S reduced Mo uptake. Since SO4-S is an anion, it can compete with Mo for the few anion-adsorption sites in the soil. Therefore, it appears that while P can increase the short-term uptake of Mo, this may be offset by any significant amount of associated SO4-S that may be present.
Silicon: There is considerable evidence with rice and sugarcane that added Si increases water-soluble and easily extractable P. Other results suggest that Si does not increase P uptake, but increases the efficiency of P use within the crop. While there is little practical application for this information for most crops, Si has been shown to improve yields of rice and sugarcane. Silicon has been shown to be effective as a disease preventative measure in rice.
Balances and Ratios
While many people believe or suspect that there are desirable ratios of P and K in the soil or a fertilizer program, research has not demonstrated that such ratios exist. It has been shown that in a few crops, there may be a desirable ratio of P with certain other elements like Zn or Ca. However, these relationships rarely play a role in modifying a fertilizer program. For now, we find the best method to evaluate P is the sufficiency range approach to both soil and plant analysis.
Plant Deficiency Symptoms
The typical visual symptoms of P deficiency exhibited by most plants include
- Stunted growth
- Dull-green or blue-green color
- Possible purple coloration on some part of the plant
- Reduced flowering and/or seed production
- Delayed maturity
Managing Phosphorus
Soil Test P Buildup
Agronomists often discuss fertilizer recommendations in terms of crop removal with or without soil test buildup as if they were unrelated subjects. In fact, they are two aspects of the same subject. A soil with a weak P level and a medium-to-strong P fixation capacity will convert or “fix” much of the applied fertilizer P into unavailable forms and leave little for the crop. In many situations, the soil will fix about 65% of the applied fertilizer P. In extreme cases, as much as 90% of applied P may be fixed. In these cases, applying only the amount of P that is expected to be removed will likely lead to a P shortage for the crop. In such soils the P fixation capacity must be overcome by higher fertilizer P rates in order to provide adequate amounts for the plants. In other words, soils with less than optimum P levels must have some soil buildup P, to insure that there is enough for crop removal. The difficulty lies in determining, with some accuracy, the minimum amount of buildup P required to overcome a particular soils fixation capacity during the season.
Soil test buildup application rates are typically discussed in terms of the ratio of applied P2O5 to soil test P buildup amounts. Often you will hear that most soils require about 9 or 10 lb. of P2O5, in excess of crop needs, in order to increase the soil test P by 1 lb (a P2O5/soil P ratio of 9 or 10). While this may be the most commonly occurring relationship, it is not nearly that simple. The range of possibilities can be quite wide and will likely be highly dependent on the initial soil test and annual rates of application.
Research at the University of Kentucky, reported in 2002, illustrates how soil test P buildup occurs. In this study, they looked at 16 different soils with initial soil test P levels ranging from 6 to 240 lb P/acre (Mehlich 3 extraction). They applied 6 different rates of phosphorous ranging from 38 to 228 lb P2O5/acre. We developed a regression equation from the Kentucky results to produce the data in Table 1. These results indicate that the initial soil P test is a major factor in determining how much P2O5 is required to buildup the soil test P.
| Table 1: Soil P Buildup Efficiency | |||
|---|---|---|---|
| Initial Soil P | lb P2O5 per lb Soil P Increase | Initial Soil P | lb P2O5 per lb Soil P Increase |
| 5 | 30.1 | 65 | 5.1 |
| 10 | 18.7 | 70 | 4.9 |
| 15 | 14.1 | 75 | 4.7 |
| 20 | 11.6 | 80 | 4.5 |
| 25 | 9.9 | 85 | 4.3 |
| 30 | 8.8 | 90 | 4.1 |
| 35 | 7.9 | 95 | 4.0 |
| 40 | 7.2 | 100 | 3.8 |
| 45 | 6.6 | 105 | 3.7 |
| 50 | 6.2 | 110 | 3.6 |
| 55 | 5.8 | 115 | 3.5 |
| 60 | 5.4 | 120 | 3.4 |
| Adapted from Thom & Dollarhide, U of KY, 2002 | |||
While other soils in different parts of the country will likely have somewhat different results, the general trend will probably be similar. Notice that when the soil P test is very low (5 to 25 lb P/ac), it requires a considerable amount of P2O5 to increase the soil test 1 lb of P/ac. However, once the soil P level is higher, the amount of P2O5 required to raise the soil P level is nearly constant. Other results suggest that many soils will reach a P2O5:soil P buildup ratio of 1:1 when the soil P test is somewhere above about 200 lb/a.
Soil Test P Drawdown
Soil test P drawdown is the “flip-side” of soil P buildup. Where a producer has high soil P tests, it makes economic and environmental sense to utilize the extra P that is “in the bank”. In theory, drawdown should work like buildup, but in reverse. Unfortunately, the results of work are not as clear or consistent as the buildup data in Table 1.
Work by Kamprath in 1964 (Table 2) illustrates that higher rates of application may also affect the efficiency of buildup. While we would expect higher rates of P2O5 to result in a higher soil test, this work also indicates that the higher rates are also more efficient (lower P2O5/P ratio).
| Table 2 |
|---|
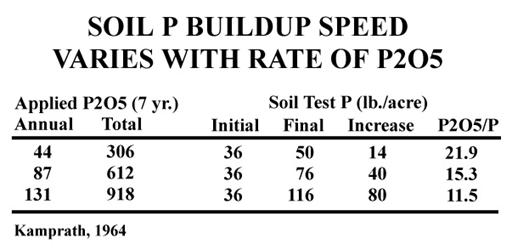 |
Another study in North Carolina (Fig 1) looked at soil P test drawdown over a long time period. In this study, the investigators applied fertilizer P by two methods. A single application of 289 lb P/ac (662 lb P2O5/ac) was compared to 8 annual banding applications prior to the beginning of the study at a rate of 54 lb P/ac each (a total of 989 lb P2O5). No additional P was applied for 26 years, while growing corn and soybeans. As seen in Fig. 1, it required between roughly 17 and 23 years to reduce the elevated soil P to the researchers designated “critical” soil P level of 44 lb P/ac. Unfortunately, the report did not document the annual yields of corn and soybeans that caused the P test drawdown. However, the nature of both curves in Fig. 1 shows that the soil test drawdown was a little faster in the initial years when the soil test was higher, than at lower levels in later years. In other words, as a soil test level approaches its “base-line” it is more difficult to change it.
| Figure 1 |
|---|
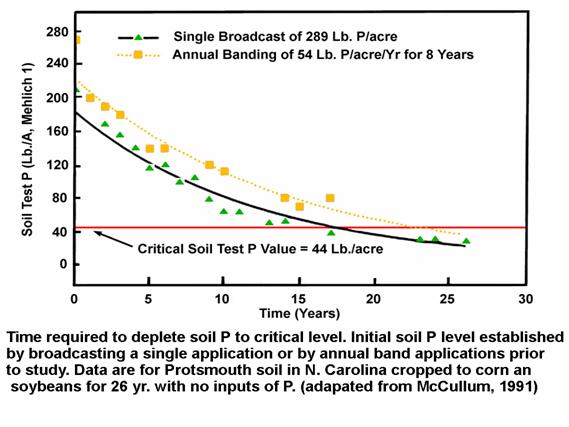 |
From this information we can conclude the following points:
- The P2O5/P buildup ratio for any soil will be higher with lower initial soil test P levels.
- Higher individual application rates of P2O5 are likely to be more efficient at increasing soil P levels than multiple smaller ones.
- Soil test P removal or drawdown will work somewhat similarly to buildup, but in reverse.
Using Phosphorus in a Fertility Program
Much has been written about P fertilizer and the relative value and benefits of different sources, placement, and timing. Since this article is intended to be relatively short and cover only the basics, it will not reprint the many pages of information on this subject. Rather, we will simply list some of the main points. Fertilizer P: For the purposes of this discussion we will consider only the most widely used sources of fertilizer P. These include the following materials.
| Name | Analysis |
|---|---|
| Diammonium phosphate (DAP) | 18-46-0 |
| Monoammonium phosphate (MAP) | variable but commonly 11-52-0 |
| Triple-Super phosphate (TSP or Triple) | 0-46-0 |
| Ammonium polyphosphate (APP or Poly) | variable but commonly 10-34-0 |
| Rock phosphate | variable |
Of these materials, DAP, MAP, and TSP are dry materials with ortho-phosphate as the primary form of P. Ammonium polyphosphate is a liquid with polyphosphate (variable length chains of ortho-phosphate) as the primary form of P. In recent years there have been few investigations into the relative effectiveness of different P sources. Work done prior to 1980 essentially settled the question by showing that the various manufactured P fertilizers (not including rock phosphate) will perform equally well under most conditions. However, within this body of work we can find results that support the superiority or inferiority of nearly all sources of P. Taken together the results indicate that for most situations, the amount of P supplied to the crop is far more significant that the source of that P. Having said that, there are still some questions about different P sources, and there are some actual differences that can be important in specific situations.
Of primary importance is how efficiently P in the fertilizer is converted into the forms that a plant can use. As stated earlier, plants can absorb only the primary (H2PO4- ) and secondary (HPO4–) ortho forms of P. Therefore, P sources must be efficiently converted to one of these two forms, if they are to benefit the crop. Remember also that the primary ortho form, which dominates in mildly acid soil, is absorbed about 10 times as efficiently as the secondary ortho form. The chemical breakdown of the major commercial P fertilizers can be illustrated as follows.
| DAP | (NH4)2HPO4 + H2O → 2NH4+ + HPO4-2 → HPO4-2 + H+ → H2PO4- |
|---|---|
| MAP | NH4H2PO4 + H2O → NH4+ + H2PO4- |
| TSP | Ca(H2PO4)2 + H2O → CaOH+ + H2PO4- |
| APP | (NH4)2H2PO7 + H2O → 2NH4+ + H2P2O7-2 → H2P2O7-2 + H2O → 2H2PO4- |
Rock Phosphate: A short discussion of rock phosphate is needed because; in times of economic stress some farmers are tempted to use this inexpensive source of phosphorus. Rock phosphate is the mineral ore used in the production of manufactured phosphorus fertilizer. There is no single mineralogical form of rock phosphate to describe the various deposits being mined around the world. However, most sources available in the U.S. are essentially insoluble in any practical time period. Given the very low solubility, rock phosphate is not recommended for crop production. Applications of typically available rock phosphate will provide little or no near-term P benefit to any crops grown in the Continental United States, or most other agriculturally advanced countries. There are a few sources of rock phosphate found in Africa that might provide some benefit within a 12 to 24 month period if they are very finely ground and applied at very much higher rates than common P fertilizers.
Liquid vs. Dry: The simple fact that a fertilizer source is in liquid or dry form has not been shown to give an agronomic advantage to one physical form over the other. Differences that may exist are typically related to the chemical composition of the fertilizer or to secondary attributes that may be related to application accuracy, timing, or other similar management factors.
Ammoniated vs. Non-Ammoniated: A significant amount of work has shown that when an ammonium form of N is in intimate contact with P, the uptake efficiency of the P is improved. The most practical and effective way to take advantage of this synergism is with ammoniated phosphate fertilizers. However, these benefits can be gained by band applications of blended or mixed applications of phosphate and ammonium N fertilizers. It appears to be generally accepted that the ratio of N:P2O5 should be no lower than 1:4 to gain any benefit from this interaction and the maximum expected benefit will often occur at an N:P2O5 ratio of about 1:1. This benefit appears to be limited to banded fertilizer applications. A band application could include both starter bands with the planter or pre-plant banding applications.
pH Effect: Diammonium phosphate (DAP), monoammonium phosphate (MAP), and ammonium polyphosphate (APP) are common ammoniated phosphorus fertilizers. While the soil pH has an effect on the availability of the P from these sources, the fertilizer also has an effect on the soil pH in the immediate area of application. In broadcast applications the variable pH effects of the different P sources occurs at all times, but any practical effects on crop performance is limited to band applications. In alkaline soils MAP and APP can have an advantage over DAP.
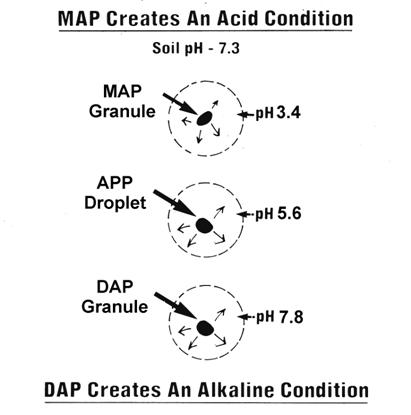  |
Polyphosphate vs. Orthophosphate: Some early work suggested an agronomic advantage for various polyphosphate fertilizers when compared to dry P fertilizers. Later work showed that this apparent advantage was related to a micronutrient contamination of the polyphosphate source. It is now generally concluded that where micronutrients are equally available, polyphosphate fertilizers can be expected to perform equally as well, but not superior to other P fertilizers.
Manure and Sludge as P Sources
The primary difference between the P supplied by organic sources such as manure and sludge, and that supplied by manufactured fertilizer, is that organic P is part of large molecules, such as protein. These large organic molecules must be broken down into ortho-phosphate before plants can use them. Most of the breakdown process is performed by bacteria and other soil organisms. The speed at which they breakdown organic molecules is primarily controlled by soil temperature and moisture. Therefore, in the year of application, little organic P is available in the spring and early summer. Another problem with organic P release is that the breakdown continues to occur into the fall, after the crop has been harvested. This late P release goes into soil test buildup (see previous discussion of soil P buildup) and can lead to environmental problems if excessive.
The amount of P in manure and sludge is highly variable, both by source and time of year. The only way to be certain of the actual nutrient content is through routine laboratory analysis. The following list contains the typical P2O5 content in common manure sources.
| Animal Type | Typical Range of P2O5 Content | |
|---|---|---|
| Percent | Lb./Ton | |
| Dairy | 0.16% - 0.25% | 3.3 - 4.9 |
| Beef | 0.34% - 0.50% | 6.7 - 10.1 |
| Veal | 0.08% - 0.13% | 1.7 - 2.5 |
| Swine | 0.43% - 0.65% | 8.6 - 13.0 |
| Sheep | 0.30% - 0.46% | 6.1 - 9.1 |
| Goat | 0.22% - 0.32% | 4.3 - 6.5 |
| Horse | 0.18% - 0.28% | 3.7 - 5.5 |
| Rabbit | 1.12% - 1.75% | 22.4 - 35.0 |
| Layers | 0.94% - 1.41% | 18.8 - 28.2 |
| Broilers | 0.67% - 1.00% | 13.4 - 20.0 |
| Turkey | 0.83% - 1.25% | 16.6 - 25.0 |
| Duck | 1.12% - 1.75% | 22.4 - 35.0 |
| Goose | 0.04% - 0.06% | 0.8 - 1.2 |
| Adapted from “Ohio livestock Manure and Wastewater Management Guide, Bulletin 604 and the North Central Region Publication No. 356, Nov. 1991 | ||
The P content of municipal sewage sludge is also highly variable, depending on the amount of P supplied by the municipality. In past years, the P content of sludge was much higher than today because most laundry detergents had a significant P content. This is not the case today. However, there are still sources of significant amounts of P that can end up in municipal sewage sludge. It has been reported (ref. 6) that municipal sewage sludge contents can range from less than 0.1% to more than 14% total P. A total P analysis does not tell us how much of that P is in a plant available form (primary or secondary orthophosphates). However, we can assume that a significant amount of this P will become available over time. Any P that is converted to the available forms is then subject to plant utilization, soil test buildup, and/or loss through erosion or drainage. Therefore municipal sewage sludge is a viable source of P for crop growth and pollution.
Recommended Rates of P2O5: Recommendations can range from 0 lb/ac to in excess of 200 lb/ac. On a very low soil P test, the upper rate of P2O5 that might be applied safely is, in most cases, beyond what most producers would be willing to spend.
Soil Testing For Phosphorus
Soil testing is used to predict the need for additional nutrients. While it is the best, and essentially only, way to predict the need for nutrient additions, it is not perfect. Nutrient uptake by plants is influenced by many factors in addition to those that can be determined by a soil test. Those soil factors that can be identified often interact in imperfectly understood ways to affect the uptake of some nutrients. Phosphorus is perhaps one of the more complicated nutrients in this respect. For many years, Researchers have been searching for better ways to predict P availability with a soil test. Because of the many investigations and the wide variety of conditions studied, we find that today there are numerous methods used to determine “available” P in a soil test. All of the commonly used soil P testing methods (see following list) are valid procedures that can serve growers well. Some of the methods have advantages or disadvantages in some situations, which are mentioned in the list. Having said this, each test can give dramatically different numerical results from others. This does not make either test wrong; it is simply the way the chemistry works. The interpretation system for each testing method is calibrated to that method, so all common procedures can be properly interpreted. Unfortunately, it is not always possible to make accurate conversions between one test result and another. In other words, if you have results from one method, it may not be possible to accurately convert that result to another method. Some conversion systems have been developed that may work well, but it is always best to work with a recent soil test in a method that you understand.
It is beyond the scope of this short paper to discuss the various methods used in soil P testing. However, some basic facts about soil P tests can be listed.
The primary accepted methods used in the U. S. include:
Bray P1: This method was developed in 1945 by Bray and Kurtz. The Bray P1 test is best suited to acid soils with a moderate CEC and base saturation, and organic soils. In soils with a pH above 7.2, the Bray P1 test may significantly underestimate the amount of available P.
Bray P2: This method was developed in 1945 by Bray and Kurtz. The Bray P1 and P2 extractants are the same in most respects, except that the hydrochloric acid in the P2 extractant is 4 times stronger than in the P1 extractant. The reason for the stronger acid in the P2 test is to extract additional soil P that exists as tricalcium phosphate. This form of P is not available to plants, and will not become available in the near future. At the time the P1 test was developed, farmers were applying large amounts of rock phosphate (tricalcium phosphate) to fields. The stronger P2 test provided an indication of the applied rock phosphate, even though that P was not available to plants. Today, few agronomists use this test to develop fertilizer recommendations.
Olsen (sodium bicarbonate): This method was developed in 1954 by S.R. Olsen as a complimentary procedure to the commonly used Bray P1 method. It provided a method of determining soil P in those alkaline soils where the Bray P1 method was unsatisfactory. Olsen-P gives a numerical result that is much smaller than the Bray P1 test and must be evaluated with its own calibration.
Mehlich 1: This method was originally introduced by Dr. Adolf Mehlich in 1953 as the North Carolina Double Acid method. It is best adapted to the coastal plain soils of the Eastern U. S. The method was subsequently renamed the Mehlich 1 method, after Dr. Mehlich developed additional soil analysis methods. The method is in use by several laboratories in East Coast States.
Mehlich 3: In 1984, Dr. Adolf Mehlich introduced the procedure now called the Mehlich 3 test. There was a Mehlich 2 procedure between numbers 1 and 3. However, this test was found to have problems and was not adopted by laboratories. The Mehlich 3 method has proven to be very efficient and well correlated with other methods, and is fast becoming the most widely used extractant for agricultural tests. It is well correlated with the Bray P1 test (r2 = 0.966) on acid soils, and on alkaline soils it is well correlated with the Olsen method (r2 = 0.918).
Morgan: The original method was introduced by Morgan in 1932. A modification of the original method is also in use. Morgan and Modified Morgan are used by a few University labs in New England and the Pacific Northwest. Both the original Morgan and the Modified Morgan are acceptable for a wide range of soil conditions. The Morgan methods tend to give very small numerical results compared to some of the other methods. The small number of labs using these methods limits their influence and usefulness on a widespread scale. Recent work at Cornell Univ., (NY) has developed a method of correlating Morgan P results with those of Mehlich 3.
Ammonium Bicarbonate-DTPA: First proposed by Soltanpour and Schwab in 1977, this method is used by a small number of laboratories. The method is highly correlated with the Olsen method for P.
Discover the leading Tricalcium Phosphate manufacturers and suppliers in India. Find trusted exporters offering high-quality TCP for pharmaceuticals, food, and industrial use.
ReplyDelete