Oxidation Reduction Potential (ORP)
Measuring the oxidation reduction potential (ORP) is such an easy, insightful test to perform, it’s a shame this poorly understood parameter is so underutilized. Of course, it’s that very lack of understanding that leaves ORP testing hidden away in the shadows. Though the science of ORP measurement is quite sophisticated, we can simplify its interpretation to serve our need to know more about a wastewater treatment system.
ORP theory is best summarized as providing an indication of the ability of a solution (the wastewater, mixed liquor suspended solids, etc.) to oxidize or reduce another solution. Add hydrogen peroxide, a strong oxidizer, to a wastewater sample, and the ORP will increase, resulting in a positive millivolt (mV) value. Add a lot of hydrogen peroxide and you will begin to oxidize some of the organics in the wastewater. Now add sodium bisulfite, a strong reducing agent, to the same sample, and the ORP will decrease, as will the oxidation of organics, and the ORP value will drop, ultimately resulting in a negative mV reading if a sufficient amount of bisulfite is added.
The chart and table shown below will provide you with a handy reference to guide your understanding of oxidation reduction potential in your wastewater system. Clicking on either image will open up a two-page PDF allowing for better viewing and high-quality printing.
ORP Measurements In the Field
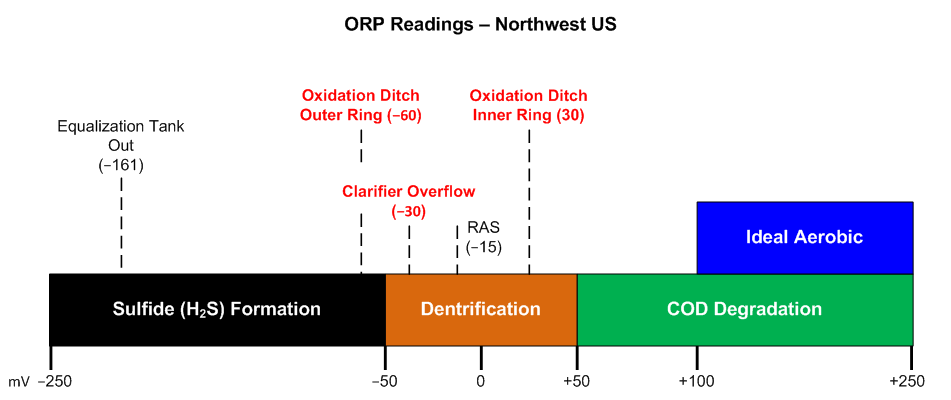
When I measure the oxidation-reduction potential at a wastewater plant I like to present the data as shown in the graphic below. This simple bar graph communicates a lot, easily and quickly, letting you know at a glance if there are any potential problems. And we do have problems at this plant, which is organically overloaded and lacking sufficient oxygen generation capacity in the bioreactor.
From the ORP measurements we can see that the wastewater leaving the equalization tank is beginning to show septicity (ORP is <-150 mV). This septicity will increase the oxygen demand in the bioreactor, which at this plant is an oxidation ditch, a schematic of which is shown below. We would like to see an ORP >50 mV in the oxidation ditch itself. Ideally, we'd like an ORP >100 mV in the bioreactor for optimal oxidation of the organics. At this heavily loaded wastewater plant the ORP is low at -60 mV in the outer ring of the oxidation ditch, not unexpected since this is where the wastewater is introduced, but the ORP remains low at just 30 mV measured at the inner ring, which is the ring where the MLSS leaves on its way to the secondary clarifiers. Once the MLSS leaves the oxidation ditch there is no additional aeration and the ORP drops to -30 mV by the time the wastewater overflows the secondary clarifiers.
Notice the installed aeration horsepower at the top right in the schematic. The rotary aerators are the standard aeration method used in oxidation ditches, contributing the majority of aeration horsepower at 340. The floating aerators, adding another 150 hp of aeration capacity. were installed to increase the air supply to better handle the high organic loading. Still, even with the added aeration, dissolved oxygen levels rarely reach 2 mg/L by the time the MLSS overflows from the oxidation ditch.
Oxidation Ditch
"Boat" Aerator
Rotary Disk Aerator
The charts and table on this page may be a little difficult to read. If you click here (or on any of the graphics above) you can open up a PDF for easier viewing/printing.
Recommended ORP Document Downloads
1. "Black magic can work wonders for wastewater treatment" from Hach wastewater specialist Mr. Bob Dabkowski.
2. ORP Management in Wastewater as an Indicator of Process Efficiency, an application note from YSI Environmental.







No comments:
Post a Comment