
Introduction
After all the many years I've been working in water and wastewater treatment it's really just this past year that I have come to better appreciate the value and importance of measuring the total kjeldahl nitrogen in addition to the ammonia nitrogen concentration entering and leaving industrial biological treatment systems. The total kjeldahl nitrogen (TKN) test measures organic nitrogen + ammonia nitrogen. Municipal wastewater treatment plants typically measure both the TKN and the ammonia concentrations at various points in their wastewater system. Industrial wastewater treatment plants, in contrast, typically measure just the ammonia concentration, missing an important contribution in ammonia loading to their biological reactor from the breakdown (conversion) of organic nitrogen to ammonia nitrogen. Nitrogen fractions are shown in Figure 1.
Figure 1: Total Nitrogen Fractions

The range of influent nitrogen concentrations entering a municipal wastewater system are shown in Table 1 below. This table is from the fourth edition of Metcalf & Eddy's "Wastewater Engineering Treatment and Reuse" textbook, a technical reference I highly recommend. Based on Table 1, the influent wastewater to a municipal treatment plant, with little or no industrial contribution, is expected to have zero nitrite and nitrate concentrations. Be aware this may not hold for industrial facilities or municipal plants with large industrial flow contributions so you need to test the influent for nitrite and nitrate in addition to organic nitrogen and ammonia to obtain a complete picture of the total nitrogen loading.
Table 1: Metcalf & Eddy Nitrogen Concentrations

Refinery Influent Nitrogen Results
The discussion that follows will be using nitrogen data generated during four days of testing at a US refinery, detailed in Table 2, below. Comparing the refinery nitrogen results in Table 2 with the M&E ranges in Table 1 above indicates that during the four days of testing the influent nitrogen concentrations ranged (approximately) between medium strength and high strength.
Table 2: Refinery Nitrogen Concentrations

Detailed Nitrogen Analysis
The influent to refinery wastewater treatment plants is typically tested for ammonia (NH3-N) but this parameter alone will often underestimate the ammonia loading to the biological system. The reason is that the ammonia test (probe or reagent method) does not measure the potentially large contribution from organic nitrogen compounds (e.g., amines) in the wastewater. With sufficient time, high wastewater temperature (a characteristic of desalter effluent) and low oxygen conditions anywhere in the wastewater system will result in the uneven conversion of organic nitrogen compounds to ammonia through hydrolysis and ammonification as illustrated in Figure 2.
Figure 2: Nitrogen Conversion

Total Kjeldahl Nitrogen
The Total Kjeldahl Nitrogen (TKN) test combines the measurement of the organic nitrogen and the ammonia nitrogen concentrations into a single value. Therefore, you must run a separate ammonia test, the value of which is subtracted from the TKN value, to obtain the organic nitrogen concentration.
For refinery biological treatment systems that experience highly variable ammonia loadings it may appear that ammonia oxidation (nitrification) has decreased when the effluent ammonia concentration increases, while this is may not actually be true. The effective oxidation of ammonia is, in a sense, hidden, due to amines and other organic nitrogen compounds being converted to ammonia after the influent sample point. When the influent TKN concentration is higher than the ammonia concentration (TKN will always be ≥ ammonia nitrogen), it will be impossible to determine nitrification efficiency because ammonia is being added to the wastewater from the breakdown of organic nitrogen at the same time ammonia is being removed (nitrified) in the bioreactor. Knowing the influent and effluent concentrations of TKN and ammonia will provide a much better understanding of how the biological system is handling (nitrifying) the applied ammonia load.
Nitrification Efficiency
What is nitrification efficiency and how can it be measured? The easiest way I know of is to calculate the nitrification rate (NR) each day as shown in Equation 1. In truth though, I have never once come across a wastewater plant that calculates the NR. The "ammonia in" value is the influent ammonia concentration measured before the bioreactor. The "ammonia out" value would typically be obtained from the secondary clarifier effluent.
Equation 1: Nitrification Rate

Several months of nitrification rate values (using refinery data) are shown below in Figure 3. There is a lot of variability in the data and there a several days where the nitrification rate dropped to zero, none of which is surprising in an industrial wastewater treatment facility.But I would offer another reason for the variability which brings us back to TKN. The nitrification rate shown in Figure 3 fails to take into account varying quantities of organic nitrogen being converted to ammonia in the bioreactor, discussed in more detail below.
Figure 3: Refinery Nitrification Rate

The graph in Figure 4 was produced using one of my favorite programs, @Risk, from the Palisade Corporation (www.palisade.com), which is an add-on program for Microsoft Excel. The histogram with the fitted curve gives another view of the variation in the nitrification rate data.
Figure 4: Distribution Fit for NR Data

TKN Results in Practice
In the graph shown in Figure 5 below, influent TKN results have been split into their respective fractions: organic nitrogen and ammonia nitrogen. It is important to realize the concentration values in the red portion of the bars represent the additional ammonia contribution from organic nitrogen not measured by the ammonia test. This additional ammonia can add a significant load to biological systems that nitrify, making it difficult to understand the variability in bioreactor dissolved oxygen concentration, pH, and alkalinity, as well as variability in effluent chemical oxygen demand, ammonia concentrations, and the nitrification rate.
Figure 5: Refinery Influent TKN Fractions

Taking a closer look at the graph in Figure 5 above, we can see that on Day 1, for example, a very high ammonia concentration of 50.7 mg/L was measured in the influent to the bioreactor. This ammonia is directly available for nitrification. But Figure 5 also shows there was, potentially, an additional 20.2 mg/L of organic nitrogen available for conversion to ammonia (amine-to-ammonia conversion) that will also need to be nitrified or it will end up in the effluent, masking (reducing) the apparent nitrification performance by reducing the overall percent removal of ammonia through the system.
On Day 2 (Figure 5, above) the unaccounted potential ammonia contribution from organic nitrogen was even greater at 34.3 mg/L. In the four sample days shown in Figure 5 there are additional ammonia contributions that are being missed by the ammonia testing, ammonia that will exert an oxygen demand in the bioreactor and make its way to the effluent in some nitrogen form.
It was stated earlier that the effective oxidation of ammonia can be hidden by failing to account for the organic nitrogen loading. The graph in Figure 6 (below), developed from the data presented above, illustrates this point. Figure 6 shows the percent reduction in ammonia by comparing the effluent ammonia in pounds per day (lb/d) to the influent TKN (lb/d) and the influent ammonia (lb/d).
Figure 6: Ammonia Reduction
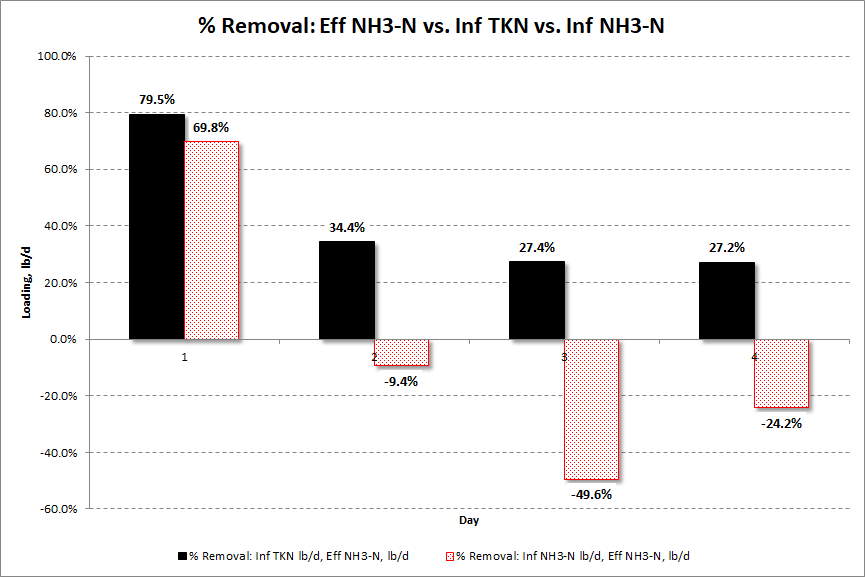
When the pounds of ammonia leaving in the effluent are compared to the influent TKN pounds, the percent reduction is positive for all four days. In contrast, using ammonia values only, after the initial ammonia slug that occurred on Day 1 the ammonia reduction is negative for the next three days. This could easily lead to the incorrect conclusion that nitrification has stopped, when it has not. Rather, having measured the TKN, the correct conclusion is that the biological system is experiencing ammonia overload but it is still nitrifying, though at a less than optimal rate. The only way we can fully understand nitrification performance is to measure the full ammonia load going to the aeration tank. The full ammonia load consists of the organic nitrogen plus the ammonia-nitrogen.
No comments:
Post a Comment