Flue gas is the combustion product gas from a fireplace, oven, furnace, boiler or steam generator that exits to the atmosphere via a ''flue'' which may be a pipe, channel or chimney. The flue is most commonly referred to as a "flue gas stack" by engineers or a "smokestack" by lay people.
Flue gases are produced when natural gas, fuel oil, coal, wood or any other fuel is combusted in an industrial furnace, a steam generator in a fossil fuel power plant or other combustion sources.
The table below provides the amount of flue gas (on a dry basis as well as a wet basis) generated by burning a typical fuel gas, fuel oil or coal. The flue gas amounts were obtained by stoichiometric calculations using the indicated typical excess combustion air percentages:

Given the amount of gas, oil or coal fuel burned in a combustion device, then the flue gas generation data (i.e., m³/GJ of fuel) in the above table provides a basis for estimating the amount of flue gas generated.
Nitrogen oxides emissions are reduced either by modifications to the combustion process to prevent their formation, or by catalytic reaction with ammonia or urea. In either case, the aim is to produce nitrogen gas, rather than nitrogen oxides.
In the United States, there is also a rapid deployment of technologies to remove mercury from flue gas - typically by adsorption on sorbents or by capture in inert solids as part of the flue gas desulfurization product.
Technologies for the removal and capture of carbon dioxide from flue gases are now under active research and development as a means of reducing the emissions of so-called ''greenhouse gas''.
Flue gases are produced when natural gas, fuel oil, coal, wood or any other fuel is combusted in an industrial furnace, a steam generator in a fossil fuel power plant or other combustion sources.
Flue gas composition
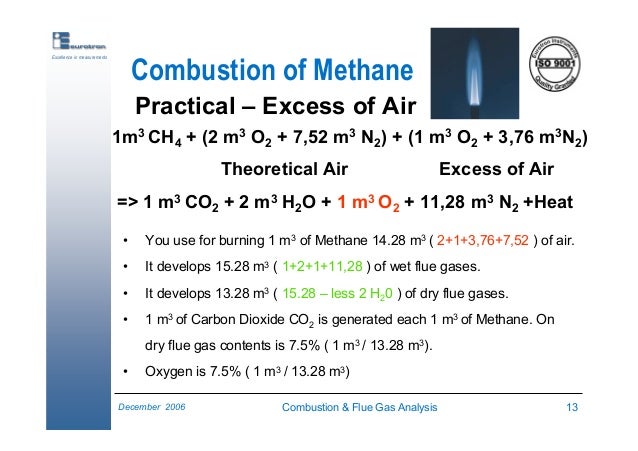
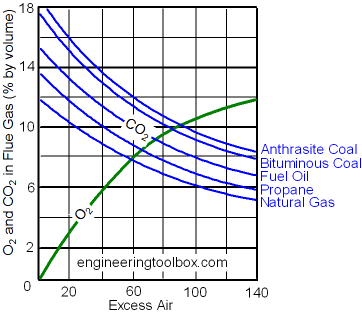
Flue gas is usually composed of carbon dioxide (CO2) and water vapor as well as nitrogen and excess oxygen remaining from the intake combustion air. It may also contain a small percentage of air pollutants such as particulate matter, carbon monoxide, nitrogen oxides, sulfur oxides and mercury. Typically, more than two-thirds of the flue gas is nitrogen.The table below provides the amount of flue gas (on a dry basis as well as a wet basis) generated by burning a typical fuel gas, fuel oil or coal. The flue gas amounts were obtained by stoichiometric calculations using the indicated typical excess combustion air percentages:

Given the amount of gas, oil or coal fuel burned in a combustion device, then the flue gas generation data (i.e., m³/GJ of fuel) in the above table provides a basis for estimating the amount of flue gas generated.
Flue gas treatment
At power plants, flue gas is often treated with a series of chemical processes and scrubbers, which remove pollutants. Electrostatic precipitators or fabric filters remove particulate matter and flue gas desulfurization removes the sulfur dioxide (SO2) produced by burning fossil fuels, particularly coal.Nitrogen oxides emissions are reduced either by modifications to the combustion process to prevent their formation, or by catalytic reaction with ammonia or urea. In either case, the aim is to produce nitrogen gas, rather than nitrogen oxides.
In the United States, there is also a rapid deployment of technologies to remove mercury from flue gas - typically by adsorption on sorbents or by capture in inert solids as part of the flue gas desulfurization product.
Technologies for the removal and capture of carbon dioxide from flue gases are now under active research and development as a means of reducing the emissions of so-called ''greenhouse gas''.






No comments:
Post a Comment