Jar test for determining “Coagulant” dosage in Water treatment
Aim:
To determine the optimum dosage of coagulant to remove small or charged particles present inside water by using “Alum” as coagulant.
Principle :
The two basic terms which can exactly explain the happenings of this experiment are “Coagulation” and “Flocculation“.
1. Coagulation: It is the process of addition of a chemical to de-stabilize a stabilized charged particle.
2. Flocculation: It is a slow mixing technique which promotes agglomeration and helps the particles to settle down.
* Generally we encounter very fine and charged clay like particles in water treatment which should be removed before we continue for further processes. These impurities do not settle by gravity when the water is passed through a sedimentation tank. The reason being that these are charged particles, they repel each other and just stay.
* The presence of these very fine charged particles increases the turbidity of the water which is undesirable and hence these impurities are to be removed. Therefore which will be using a chemical which dissociates as soon as it added to water and helps in the process of “Coagulation”. In the present experiment we are using “Alum” [Al2(SO4)3. 18H2O] as the clarifying agent.
*When alum solution is added to water, the molecules dissociate to yield SO4^2- and Al3+. These charged species combine with the charged colloidal particles to neutralize the charge. A detailed explanation of the charge removal can be found in the web which will be based on two basic definitions “Stern potential“ and “Zeta Potential“.
* Through the slow mixing or so called “Flocculation” a process known as agglomeration occurs which combines the charged particles into a compact whole and helps in the settling of the particle. That is the reason why we have step of “slow mixing” in the present experiment.
Apparatus:
1. Jar testing apparatus
2. Turbidity meter
3. Beaker, burette, pipette
Reagents required: Alum solution (1 ml containing 10 mg of alum)
Procedure :
1. Take 1000 ml of given sample in 6 beakers.
2. Find the pH of the sample and adjust it to 6 to 8.5.
3. Now add 1 ml, 2 ml, 4 ml, 8 ml, 10 ml, 12 ml of alum respectively in each one of the beakers.
4. Now insert the paddle of the jar testing apparatus inside the beakers and start it.
5. Initially maintain a speed such that the paddles rotate at an angular velocity of 100 rpm for a time of 1 minute.
6. Now adjust the speed such that the paddles rotate at 40 rpm/min for a time of 9 minutes.
7. Now allow the beakers to settle down for 10 minutes.
8. Make an observation as of which of the 6 beakers is most clearer. Also measure the turbidity of each beaker using a turbidity meter and tabulate your results.
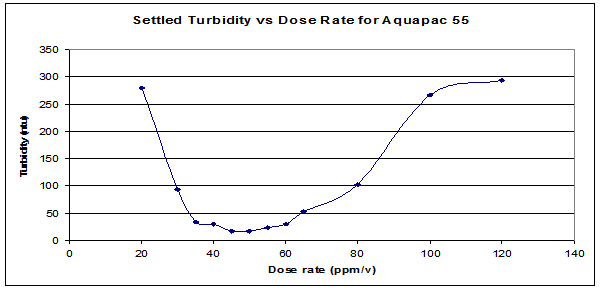
9. Plot a graph “Settled turbidity” Vs “Coagulant dosage”.
Result:
The optimum value of coagulant dosage from the graph should be reported.
* The optimum value of coagulant generally lies between 8 to 10 for normal water from rivers.
The graph varies as shown (only shape not exact graph)

I enjoyed reading this blog. in my opinion, everything was perfectly written there as well a few small tips are also can be taken as a healthy suggestion. Descriptive informative content written in this blog is very useful. Plant wastewater treatment manufacturer
ReplyDelete