How to Select Oxidation Reduction Potential (ORP) Instruments



Analyzer for ORP. Hand-held ORP meter. Analog and digital display ORP monitor.
Image Credit: ABB | Omega | + GF +
Oxidation reduction potential (ORP) instruments, also
known as redox potential instruments, are used to monitor chemical
reactions. ORP instruments measure the ability of a solution to act as
an oxidizing agent, and to quantify ion activity. It is a rugged
electrochemical test, which is convenient and easy to use. The test is
often accomplished using an in-line and handheld instrument which
provides a consistent and reliable measurement. Oxidation reduction
potential measurements should be used as a range of operation rather
than a single point reading. There are disadvantages to the testing
instruments including carryover of the electrodes and the inability to
distinguish the effects of pH and temperature. The speed of an ORP
instrument is directly related to the exchange current density, derived
from concentration, the oxidation reduction system, and the electrode.
The speed diminishes when the sample ORP is similar to the electrode
ORP. The listed disadvantages can be compensated for by rinsing the
instrument's electrodes before readings and by correlating the system by
checking the oxidizer and reducer in a steady state system with a wet
test, and measuring pH.
Oxidation Reduction Reactions
Oxidation reduction potential is a measurement of the
electrical potential of a redox reaction. It also serves as a measure
of how much oxidation or reduction takes place in the given conditions.
Redox reaction refers to the exchange of electrons. Oxidation is the
loss of electrons and therefore the solution is more positive. Reduction
is the gaining of electrons which causes a negative charge. The two
processes always occur together because the oxidizing agent during
reduction. The potential, measured by the ORP instruments, is a measure
of the power of a substance to gain electrons in solution. The stronger
the reducing agent, the more likely it will readily lose electrons and
give them to another substance. A strong reducing agent will have a high
negative redox potential. A high positive redox potential is associated
with a strong oxidizing agent. The ORP measurement is not a measurement
of concentration; rather it measures the activity level, similar to a
pH test. The basic thermodynamics for ORP reactions can be expressed
using the Nerest equation.
Ecell= E°Ox/Red+2.303RT/nF [Ox/Red]
Where,
E° = potential under standard conditions of unit activity referred to the standard hydrogen electrode.
R = the gas constant, 1.986 cal, per mol. degree
F = Faraday's constant
T = temperature in K
n = the number of electrons exchanged in the reaction.
E°Ox/Red is found in tables in handbooks and is the value relative to the standard hydrogen electrode (SHE).
How Oxidation Reduction Potential Instruments Work
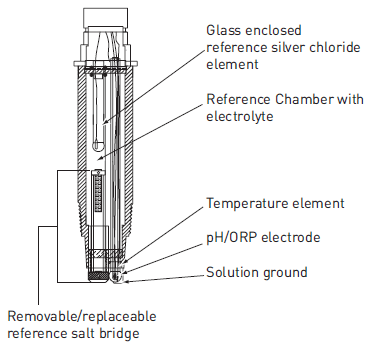
ORP/ pH electrode. Image Credit: gfsignet.com
Oxidation reduction potential (ORP) instruments are
small electrical devices. The device consists of a metal half-cell and a
reference cell. When the measuring electrode is exposed to oxidizing or
reducing agents, electrons are constantly transferred back and forth on
its measuring surface which generates a tiny voltage. The measuring or
sensing electrode is made of platinum, gold, or graphite. The reference
electrode is connected to a salt water solution and given a half cell
potential of 0.0mV. This electrode is made of silver chloride or
saturated calomel (SCE) because they provide stable and reliable
performance. Reduction potentials are defined relative to a reference
node because absolute potentials are hard to accurately measure. The
device used to measure ORP is very similar to the device used to measure
pH. The only difference is the ORPs rarely have a temperature
compensation mechanism. The ORP electrodes measure the voltage across a
circuit formed by the two electrodes and the measurement can be made
using the millivolt mode of a pH meter. A single voltage is called the
oxidation-reduction potential, where a positive voltage shows a solution
attracting electrons (oxidizing agent). ORP instruments can measure
oxidation reduction potential in the range of -450 to +1100 mV. Readings
toward the positive region of this range by these instruments indicate a
strong oxidizing agent, while readings toward the negative region by
indicate a strong reducing agent. The chart below shows standard
conditions for reduction potentials.
Standard Reduction Potential
|
eo (V)
|
Ac3+ + 3e « Aco
|
-2.20
|
Ag+ + e « Ago
|
0.7996
|
Ag2MoO4 + 2e « 2Ago + MoO42-
|
0.4573
|
Ag2S + 2e « 2Ago + S2-
|
-0.691
|
H2BO3- + 5H2O + 8e ® BH4- + 8OH-
|
-1.24
|
H2BO3- + H2O + 3e ® Bo + 4OH-
|
-1.79
|
Bi+ + e ® Bio
|
0.5
|
Be3+ + 3e ® Bio
|
0.308
|
2HCNO + 2H+ + 2e « (CN)2 + 2H2O
|
0.330
|
(CNS)2 + 2e « 2CNS-
|
0.77
|
CO2 + 2H+ + 2e « HCOOH
|
-0.199
|
HCHO + 2H2O + 2e « CH3OH + 2OH-
|
-0.59
|
Standard Reduction Potentials. Chart Adapted from Northland College
The general process for preforming an oxidation reduction potential test consists of six steps described in the image below.
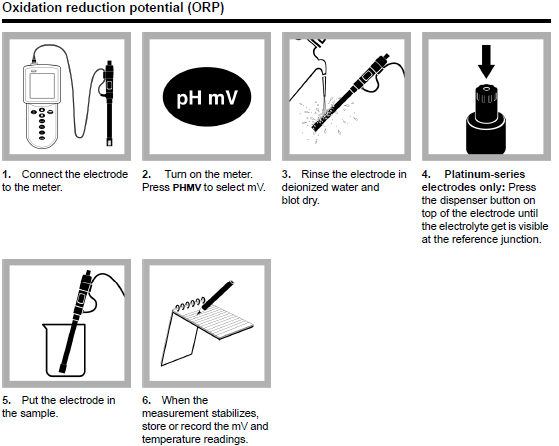
Image Credit: HACH
When selecting an oxidation reduction potential
instrument there are several important factors to consider. They are
described in the video and selection guide below.
Basic tips for choosing the proper instrument for water quality tests.
Video Credit: MyronLMeters
Mounting
The oxidation reduction potential instrument is
available in several mounting options to fit the desired application.
ORP tests are frequently done in laboratories as well as in remote water
storage units or water supplies, therefore the mounting and portability
are important to consider when selecting an instrument.
-
Hand held devices are designed to be operated while held by hand. They are rarely able to establish a fixed point in a system and the "bounce" observed measurement may be as much as 25mV. This depends on if the device is stationary or in motion. The University of California Division of Agriculture and Natural Resources recommends that handheld ORP instruments be held in the source for 30 seconds.
-
Portable devices have handles, a case, or wheels for ease of movement. They are not necessarily handheld.
-
Modular devices are modular and can be interfaced with sensors or input ranges.
-
Lab / benchtop devices are designed to sit atop a bench or desktop in a laboratory. They are not suitable for field use.
-
In-Situ / field devices are designed for in-situ or field use, outside of a laboratory setting. They are made of durable materials that can withstand weather conditions.
-
Panel devices attach to a panel in a laboratory or water supply tank
Often the pH measurement device and the ORP instrument can be mounted to the same line in storage tanks.
ORP Measurement
- ORP / redox range- The ORP / redox range the instrument is capable of measuring. As seen in the chart below, reactions produce a positive or negative ORP depending on the type and quality of the reaction occurring. The oxidation scale can go from about -1000 to +1000. Sources with a strong negative ORP are safer to consume.
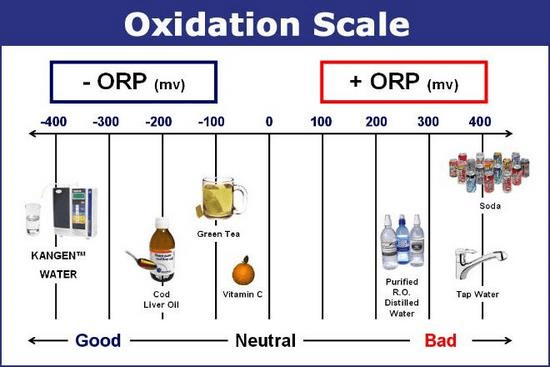
Oxidation scale. Image credit: Kangen Water
- Accuracy- The ORP / redox accuracy of the instrument (±mV). The size of the electrode influences the fluctuations in readings. A larger detection area means a better sensor. To test the accuracy of the test, the measurement can be taken in the oxidative and was as reductive directions. If the curves produced by the data collected overlap, it can be assumed that the reaction was carried out while the electrode was in equilibrium.
-
ORP reference electrode input- The instrument has an ORP reference electrode input. The reference node should be placed in a saline solution. ORP measurements provide a window based on the reference node.
pH Measurement
Oxidation reduction potential instruments are very
similar to pH measurement devices. They are often in the same device and
produce voltages that depend on the solutions in contact with their
sensing electrodes. pH electrodes are designed to produce 0 mV at pH 7
(neutral), positive mV below pH 7 (acidic), a negative mV above pH 7
(basic).
-
Measures pH- The instrument measures pH.
-
pH range- The pH range the instrument is capable of measuring.
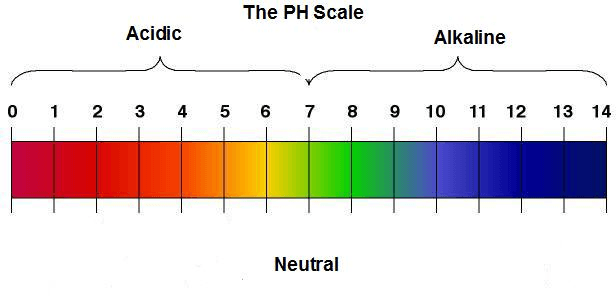
pH scale. Image Credit: Abundant Health Center
-
Accuracy- The pH accuracy of the instrument (±pH).
-
pH reference electrode input- The pH instrument has a reference electrode input.
Instrument Specific Options
-
Controller- Devices provide some controller functionality, such as set limits and proportional (P) control, proportional integrative (PI) control, or proportional integrative derivative (PIC) control.
-
Control relays- The number of control relays. A relay is an electrical switch that opens and closes under control another electrical circuit. Relays use the ORP level to do several functions including, switching on an alarm or indicator, triggering the device to restore acceptable levels, or creating a data log indicating when the relay was tripped. The number of relays will indicate the number of switches and therefore functions the device can complete.
-
Temperature measurement- The instrument also measures / outputs temperature. ORP instruments have an on-board temperature measurement device for rapid measurements.
-
Temperature compensation- Temperature compensation is very important for an accurate pH measurement; however, it is not used for ORP measurement. Temperature does affect the reaction potentials for all chemicals but true ORP is the direct measurement of electrons moving during an oxidation-reduction reaction, regardless of temperature.
-
Process media temperature- The temperature of the media in which the sensor, probe, or electrode is immersed. It is important to be aware of the ambient temperature and the temperature of the process for proper calibration.
-
Operating temperature- The temperature range over which devices are designed to operate.
-
- Battery powered- Devices require batteries for full operation, not just a battery backup. Batteries allow the device to be portable and handheld for use in the field.
-
Filters- Devices provide special signal processing or filtering for clean, easy to use data.
-
Event triggered- Devices can capture and/or log data when an event happens. Events such as the addition or subtraction of a chemical, change in process temperature, or introduction of the device into a process will automatically trigger the ORP instrument to start a measurement.
-
Built-in or self-calibration- Devices have a built-in calibrator, which can be used for testing and readjustment.
-
Extreme environment- Devices are designed to withstand harsh environments with extreme humidity, temperature, dust, etc.
-
Self-test / diagnostics- Devices have self-test or diagnostics capabilities. Some devices may have warnings or reminders for rinsing between readings.
User Interface
The user interface refers to several critical design
features of an oxidation reduction potential instrument. A simple, well
designed user interface will affect the usability, train-ability, and
overall satisfaction with the device. User
interface considerations include the display and controls.
Display Type
The display is what is used by the user to see the measurement.
-
Analog meter- Devices have an analog meter, typically with a needle and a face with values corresponding to the measurement range.
-
Digital display- Devices have a digital display generally with an LED screen.
-
Video display- Devices have a cathode ray tube (CRT), liquid-crystal display (LCD), flat panel display (FPD), or other multi-line display for video monitoring of a system or process.
-
None- Devices do not have a display and may serve as an alarm or indicator if the measurement goes outside of its desired range.
User Controls
User controls dictate how the user will interact with the machine.
-
Manual controls- Devices have simple, front panel or local controls such as knobs and/or potentiometers.
-
Digital front panel- Devices have a digital front panel that is programmed with a keypad. Digital panels may be equipped to complete a higher level of analysis and be easier to interpret, however, they may be more complicated to carry and use.

Digital front panel. Image Credit: Yokogawa
-
Computer interface- Devices are programmed with a computer interface. Computer interfaces have similar properties to digital panels.
-
None- Devices do not have user controls.
Output Options
Output is interpreted by the user in order to evaluate the ORP of the application.
Electrical Output
-
Analog voltage- The output is an analog voltage. This is a simple, usually linear, function of the measurement.
-
Analog current- The output is an analog current that is imposed on the output circuit proportional to the measurement. Feedback provides the appropriate current, regardless of variables such as line noise and impedance. Analog current outputs or transmitters are often used to send signals over long distances.
-
Analog frequency- The output is an analog frequency, a format which transmits information with continuous physical variables such as voltage amplitude or frequency variations.
-
Switch / alarm relay- The output is a change-in-state of a switch or alarm.
Interface Options
-
Serial- A standard digital output protocol (serial) such as RS232, RS422, RS485, etc.
-
Parallel- A standard digital output protocol (parallel) such as IEEE 488, etc.
-
Other- Any digital output other than the standard serial or parallel signals. Simple TTL logic signals are an example.
Special Features
Some special features of oxidation reduction potential instruments include:
-
Heavy duty- If the instrument is going to be used in the field it should be durable enough to handle the environmental conditions by being waterproof and UV resistant.
-
In-line- In-line devices can be mounted or installed in the system, such as in a pipe or tank the fluid goes through. They may be programmed to take continuous readings to alert the user if there is a change in the process.
-
Low noise- The device can be used when noise is of concern such as in a laboratory.
-
Wireless- Wireless devices are able to transmit data remotely to the home base for further analysis.
-
Flame resistant- ORP instruments used in a laboratory or in the field may face hazardous conditions such as fire due to chemicals.
Uses
Oxidation reduction potential (ORP) instruments are
mainly used to measure the oxidizing ability of chlorine in swimming
pools and spas, and to determine when the equivalence point has been
reached in an oxidation-reduction reaction. ORP instruments are also
used for cooling tower disinfection, groundwater remediation, bleaching,
cyanide destruction, chrome reductions, metal etching, fruit and
vegetable disinfection, and dechlorination.
Chemicals like chlorine, bromine, and ozone are all
strong oxidizers. Because they are able to take electrons from other
substances they are excellent sanitizers. The act of sanitization is
caused by the alteration of the chemical makeup of the unwanted
organism. As the oxidizers are reduced, their ability to oxidize is
reduced until they are used up or combined with another substance. The
oxidation process can be monitored by an ORP instrument and the user can
identify the end of the sanitization process. Oxidation reduction
potential instruments can possibly select an individual ORP value,
expressed in millivolts, at which a predictable level of disinfection
can be achieved and sustained regardless of variations in either oxidant
demand or oxidant concentration.

ORP water source field test. Image Credit: Wittenberg University
Oxidation reduction potential instruments have proven
their use as analytical tools in monitoring changes in a system rather
than determining their absolute value (e.g., process control and
titrations).
Standards
The value of ORP measurements has been popular since
the 1960s as a way to gauge water quality. In 1971, the World Health
Organization set a standard ORP value for drinking water disinfection at
650 millivolts. This means that when a body of water measures 650/1000
(about 2/3) of a volt, the sanitizer in the water is active enough to
destroy harmful organisms almost instantaneously.
Thanks for sharing nice information with us. I like your post and all you share with us is upto date and quite informative, you have done a wonderful job negative ORP
ReplyDelete