What is Acid Rain?
Acid rain, or acid deposition, is a broad term that includes any form of precipitation with acidic components, such as sulfuric or nitric acid that fall to the ground from the atmosphere in wet or dry forms. This can include rain, snow, fog, hail or even dust that is acidic.
What Causes Acid Rain?
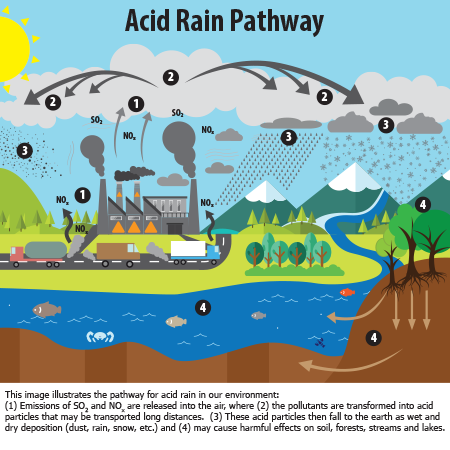 Acid rain results when sulfur dioxide (SO2) and nitrogen oxides (NOX) are emitted into the atmosphere and transported by wind and air currents. The SO2 and NOX react with water, oxygen and other chemicals to form sulfuric and nitric acids. These then mix with water and other materials before falling to the ground.
Acid rain results when sulfur dioxide (SO2) and nitrogen oxides (NOX) are emitted into the atmosphere and transported by wind and air currents. The SO2 and NOX react with water, oxygen and other chemicals to form sulfuric and nitric acids. These then mix with water and other materials before falling to the ground.
While a small portion of the SO2 and NOX that cause acid rain is from natural sources such as volcanoes, most of it comes from the burning of fossil fuels. The major sources of SO2 and NOX in the atmosphere are:
- Burning of fossil fuels to generate electricity. Two thirds of SO2 and one fourth of NOX in the atmosphere come from electric power generators.
- Vehicles and heavy equipment.
- Manufacturing, oil refineries and other industries.
Winds can blow SO2 and NOX over long distances and across borders making acid rain a problem for everyone and not just those who live close to these sources.
Forms of Acid Deposition
Wet Deposition
Wet deposition is what we most commonly think of as acid rain. The sulfuric and nitric acids formed in the atmosphere fall to the ground mixed with rain, snow, fog, or hail.
Dry Deposition
Acidic particles and gases can also deposit from the atmosphere in the absence of moisture as dry deposition. The acidic particles and gases may deposit to surfaces (water bodies, vegetation, buildings) quickly or may react during atmospheric transport to form larger particles that can be harmful to human health. When the accumulated acids are washed off a surface by the next rain, this acidic water flows over and through the ground, and can harm plants and wildlife, such as insects and fish.
The amount of acidity in the atmosphere that deposits to earth through dry deposition depends on the amount of rainfall an area receives. For example, in desert areas the ratio of dry to wet deposition is higher than an area that receives several inches of rain each year.
Measuring Acid Rain
 Acidity and alkalinity are measured using a pH scale for which 7.0 is neutral. The lower a substance's pH (less than 7), the more acidic it is; the higher a substance's pH (greater than 7), the more alkaline it is. Normal rain has a pH of about 5.6; it is slightly acidic because carbon dioxide (CO2) dissolves into it forming weak carbonic acid. Acid rain usually has a pH between 4.2 and 4.4.
Acidity and alkalinity are measured using a pH scale for which 7.0 is neutral. The lower a substance's pH (less than 7), the more acidic it is; the higher a substance's pH (greater than 7), the more alkaline it is. Normal rain has a pH of about 5.6; it is slightly acidic because carbon dioxide (CO2) dissolves into it forming weak carbonic acid. Acid rain usually has a pH between 4.2 and 4.4.
Policymakers, research scientists, ecologists, and modelers rely on the National Atmospheric Deposition Program’s (NADP) National Trends Network (NTN) for measurements of wet deposition. The NADP/NTN collects acid rain at more than 250 monitoring sites throughout the US, Canada, Alaska, Hawaii and the US Virgin Islands. Unlike wet deposition, dry deposition is difficult and expensive to measure. Dry deposition estimates for nitrogen and sulfur pollutants are provided by the Clean Air Status and Trends Network (CASTNET). Air concentrations are measured by CASTNET at more than 90 locations.
When acid deposition is washed into lakes and streams, it can cause some to turn acidic. The Long-Term Monitoring (LTM) Network measures and monitors surface water chemistry at over 280 sites to provide valuable information on aquatic ecosystem health and how water bodies respond to changes in acid-causing emissions and acid deposition.
https://www.epa.gov/acidrain/what-acid-rain
No comments:
Post a Comment