Introduction
Acidic pollutants can be deposited from
the atmosphere to the Earth's surface in wet and dry
forms. The common term to describe this process is acid
deposition. The term acid
precipitation is used to specifically describe
wet forms of acid pollution that can be found in rain,
sleet, snow, fog, and cloud vapor. An acid can be defined
as any substance that when dissolved in water dissociates
to yield corrosive hydrogen ions. The acidity of substances
dissolved in water is commonly measured in terms of pH (defined
as the negative logarithm of the concentration of hydrogen
ions). According to this measurement scale solutions
with pHs less than 7 are described as being acidic,
while a pH greater than 7.0 is considered alkaline (Figure
8h-1). Precipitation normally has a pH between 5.0
to 5.6 because of natural atmospheric reactions involving
carbon dioxide. For comparison, distilled water, pure
of any other stubstances, would have a pH of 7.0. Precipitation
is considered to be acidic when its pH falls below 5.6
(which is 25 times more acidic than pure distilled water).
Some sites in eastern North America have precipitation
events with pHs as low as 2.3 or about 1000 times more
acidic than natural.
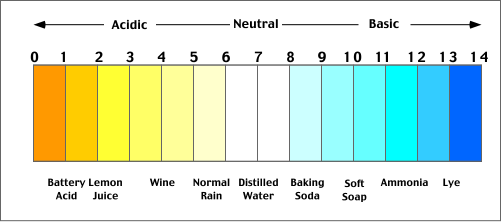
| Figure 8h-1: The
pH scale. A value of 7.0 is considered neutral.
Values higher than 7.0 are increasingly alkaline
or basic. Values lower than 7.0 are increasingly
acidic. The illustration above also describes the
pH of some common substances. |
Acid deposition is not a recent phenomenon. In the 17th century, scientists
noted the ill effects that industry and acidic pollution was having on vegetation
and people. However, the term acid
rain was first used two centuries later when Angus Smith published
a book called 'Acid Rain' in 1872. In the
1960s, the problems associated with acid deposition became an international
problem when fishermen noticed declines in fish numbers and diversity in many
lakes throughout North America and Europe.
Acid Deposition Formation
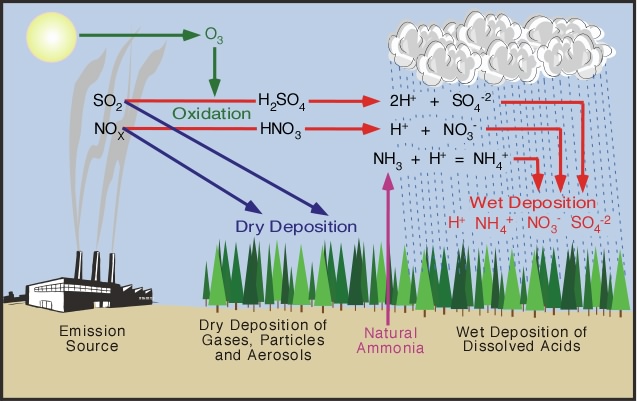
Acid deposition can form as a result of
two processes. In some cases, hydrochloric acid can be
expelled directly into the atmosphere. More commonly
it is due to secondary
pollutants that form from the oxidation of nitrogen
oxides (NOx) or sulfur
dioxide (SO2) gases that
are released into the atmosphere (see Figure
8h-2). Reactions at the Earth's surface or
within the atmosphere can convert these pollutants into nitric
acid or sulfuric
acid. The process of altering these gases into
their acid counterparts can take several days, and during
this time these pollutants can be transported hundreds
of kilometers from their original source. Acid precipitation
formation can also take place at the Earth's surface
when nitrogen oxides and sulfur dioxide settle on the
landscape and interact with dew or frost.
Emissions of sulfur dioxide are responsible
for 60-70% of the acid deposition that occurs globally.
More than 90% of the sulfur in the atmosphere is of
human origin. The main sources of sulfur include:
- Coal burning - coal typically contains 2-3% sulfur
so when it is burned sulfur dioxide is liberated.
- The smelting of metal sulfide ores to obtain the
pure metals. Metals such as zinc, nickel, and copper
are all commonly obtained in this manner.
- Volcanic eruptions - although this is not a widespread
problem, a volcanic eruption can add a lot of sulfur
to the atmosphere in a regional area.
- Organic decay.
- Ocean spray.
After being released into the atmosphere,
sulfur dioxide can either be deposited on the Earth's
surface in the form of dry deposition or it can undergo
the following reactions to produce acids that are incorporated
into the products of wet deposition (Figure 8h-2):
SO2 + H2O »»» H2SO3
H2SO3 + 1/2O2 »»» H2SO4
|
Figure 8h-2: Several
processes can result in the formation of acid
deposition. Nitrogen oxides (NOx) and sulfur
dioxide (SO2) released
into the atmosphere from a variety of sources
call fall to the ground simply as dry deposition.
This dry deposition can then be converted into
acids when these deposited chemicals meet water.
Most wet acid deposition forms when nitrogen
oxides (NOx) and sulfur dioxide (SO2)
are converted to nitric
acid (HNO3) and sulfuric
acid (H2SO4)
through oxidation and dissolution. Wet deposition can
also form when ammonia gas
(NH3) from natural sources
is converted into ammonium (NH4).
|
Some 95% of the elevated levels of nitrogen
oxides in the atmosphere are the result of human activities.
The remaining 5% comes from several natural processes.
The major sources of nitrogen oxides include:
- Combustion of oil, coal, and gas.
- Bacterial action in soil.
- Forest fires.
- Volcanic action.
- Lightning.
Acids of nitrogen form as a result of the
following atmospheric chemical reactions (see Figure
8h-2 above):
NO + 1/2O2 »»» NO2
2NO2 + H2O »»» HNO2 +
HNO3
NO2 + OH »»» HNO3
Finally, the concentrations of both nitrogen
oxides and sulfur dioxides are much lower than atmospheric
carbon dioxide which is mainly responsible for making
natural rainwater slightly acidic. However, these gases
are much more soluble than carbon dioxide and therefore
have a much greater effect on the pH of the precipitation.
Effects of Acid Deposition
Acid deposition influences the environment
in several different ways. In aquatic systems, acid deposition
can effect these ecosystems by lowering their pH. However,
not all aquatic systems are effected equally. Streams,
ponds, or lakes that exist on bedrock or sediments rich
in calcium and/or magnesium are naturally buffered from
the effects of acid deposition. Aquatic systems on neutral
or acidic bedrock are normally very sensitive to acid
deposition because they lack basic compounds that buffer
acidification (see Figure 8h-3). In Canada, many
of the water bodies found on the granitic Canadian
Shield fall in this group. One of the most obvious
effects of aquatic acidification is the decline in fish
numbers. Originally, it was believed that the fish died
because of the increasing acidity of the water. However,
in the 1970s scientists discovered that acidified lakes
also contained high concentrations of toxic heavy metals
like mercury, aluminum, and cadmium. The source of these
heavy metals was the soil and bedrock surrounding the
water body. Normally, these chemicals are found locked
in clay particles, minerals, and rocks. However, the
acidification of terrestrial soils and bedrock can cause
these metals to become soluble. Once soluble, these toxic
metals are easily leached by infiltrating water into
aquatic systems where they accumulate to toxic levels.
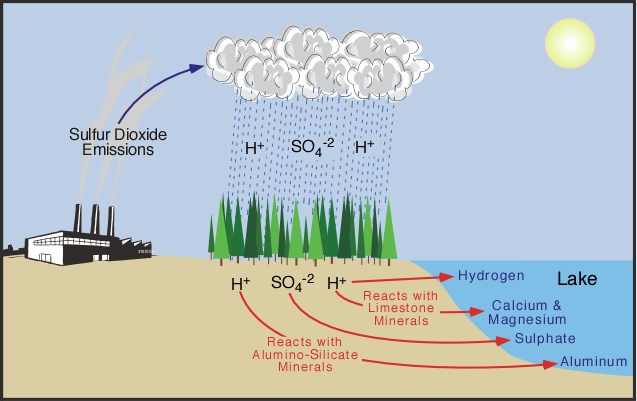
| Figure 8h-3: Lake
acidification begins with the deposition of the
byproducts acid precipitation (SO4 and
H ions) in terrestrial areas located adjacent to
the water body. Hydrologic processes then move
these chemicals through soil and bedrock where
they can react with limestone and aluminum-containing
silicate minerals. After these chemical reactions,
the leachate continues
to travel until it reaches the lake. The acidity
of the leachate entering lake is controlled by
the chemical composition of the effected lake's
surrounding soil and bedrock. If the soil and bedrock
is rich in limestone the acidity of the infiltrate
can be reduced by the buffering action of calcium
and magnesium compounds. Toxic aluminum (and some
other toxic heavy metals) can leach into the lake
if the soil and bedrock is rich in aluminum-rich
silicate minerals. |
In the middle latitudes, many acidified aquatic systems experience a phenomenon
known as acid shock.
During the winter the acidic deposits can buildup in the snowpack. With the
arrival of spring, snowpack begins to melt quickly and the acids are released
over a short period of time at concentrations 5 to 10 times more acidic than
rainfall. Most adult fish can survive this shock. However, the eggs and small
fry of many spring spawning species are extremely sensitive to this acidification.
The severity of the impact of acid deposition on vegetation is greatly dependent
on the type of soil the plants grow in. Similar to surface water acidification,
many soils have a natural buffering capacity and are able to neutralize acid
inputs. In general, soils that have a lot of lime are better at neutralizing
acids than those that are made up of siliceous sand or weathered acidic bedrock.
In less buffered soils, vegetation is effected by acid deposition because:
- Increasing acidity results in the leaching of several
important plant nutrients, including calcium, potassium,
and magnesium. Reductions in the availability of
these nutrients cause a decline in plant growth rates.
- The heavy metal aluminum becomes more mobile in
acidified soils. Aluminum can damage roots and interfere
with plant uptake of other nutrients such as magnesium
and potassium.
- Reductions in soil pH can cause germination of
seeds and the growth of young seedlings to be inhibited.
- Many important soil organisms cannot survive is
soils below a pH of about 6.0. The death of these
organisms can inhibit decomposition and nutrient
cycling.
- High concentrations of nitric acid can increase
the availability of nitrogen and reduce the availability
of other nutrients necessary for plant growth. As
a result, the plants become over-fertilized by nitrogen
(a condition known as nitrogen
saturation).
- Acid precipitation can cause direct damage to the
foliage on plants especially when the precipitation
is in the form of fog or cloud water which is up
to ten times more acidic than rainfall.
- Dry deposition of SO2 and
NOx has been found to affect the ability of leaves
to retain water when they are under water stress.
- Acidic deposition can leach nutrients from the
plant tissues weakening their structure.
The combination of these effects can lead
to plants that have reduced growth rates, flowering ability
and yields. It also makes plants more vulnerable to diseases,
insects, droughts and frosts.
The effects of acidic deposition on humans can be divided into three main categories.
Acid deposition can influence human health through the following methods:
- Toxic metals, such as mercury and aluminum, can
be released into the environment through the acidification
of soils. The toxic metals can then end up in the
drinking water, crops, and fish, and are then ingested
by humans through consumption. If ingested in great
quantities, these metals can have toxic effects on
human health. One metal, aluminum, is believed to
be related to the occurrence of Alzheimer's
disease.
- Increased concentrations of sulfur dioxide and
oxides of nitrogen have been correlated to increased
hospital admissions for respiratory illness.
- Research on children from communities that receive
a high amount of acidic pollution show increased
frequencies of chest colds, allergies, and coughs.
Acid deposition also influences the economic
livelihoods of some people. Many lakes and streams on
the eastern coast of North America are so acidic that
the fish decline significantly in numbers. The reduced
fish numbers then influence commercial fishermen and
industries that rely on sport fishing tourism. Forestry
and agriculture are effected by the damage caused to
vegetation. In some areas of eastern North America and
Europe, large die-backs of trees have occurred.
Finally, acid deposition effects a number inanimate features of human construction.
Buildings and head stones that are constructed from limestone are easily attacked
by acids, as are structures that are constructed of iron or steel. Paint on
cars can react with acid deposition causing fading. Many of the churches and
cathedrals in Europe are under attack from the effects of acidic deposition.


No comments:
Post a Comment