(a). Characteristics of Energy and Matter
|
|
Introduction
Energy is
defined simply by scientists as the capacity for doing
work. Matter is
the material (atoms and molecules)
that constructs things on the Earth and in the Universe.
Albert Einstein suggested early in this century that
energy and matter are related to each other at the atomic
level. Einstein theorized that it should be possible
to convert matter into energy. From Einstein's theories,
scientists were able to harness the energy of matter
beginning in the 1940s through nuclear fission.
The most spectacular example of this process is a nuclear
explosion from an atomic bomb. A more peaceful example
of our use of this fact of nature is the production of
electricity from controlled fission reactions in nuclear
reactors. Einstein also suggested
that it should be possible to transform energy into
matter.
Energy and matter are also associated to
each other at much larger scales of nature. Later on
in this chapter, we will examine how solar radiation
provides the energy to create the matter that makes up
organisms. Organisms then use some of this matter to
power their metabolism.
Types of Energy
Energy comes
in a variety of forms. The simplest definition of the
types of energy suggests that two forms exist: kinetic
energy and potential
energy. Kinetic
energy is the energy due to motion. A rock falling from
a cliff, a bee in flight, wind blowing leaves of trees,
and water following over a waterfall are all examples
of kinetic energy. Potential energy is the energy stored
by an object that can be potentially transformed into
another form of energy. Water stored behind a dam, the
chemical energy of the food we consume, and the gasoline
that we putting in our cars are all examples of potential
energy. Conversion of this energy occurs when the energy
in food is used by an organism to energize its metabolism,
when the water in the dam flows through turbines to produce
electricity from motion, and when the gasoline is used
in a engine to produce motion from combustion.
Some other forms of energy include heat,
electricity, sound, energy of chemical reactions, magnetic
attraction, energy of atomic reactions, and light. Definitions
for a few of these types of energy are as follows:
Radiation -
is the emission of energy from a material object in
the form of electromagnetic
waves and photons.
Chemical
Energy - is the energy produced or consumed
in chemical reactions.
Atomic
Energy - is the energy released from an
atomic nucleus because of a change in its subatomic mass.
Electrical
Energy - is
the energy produced from the force between two
objects having the physical property of electrical
charge.
Heat
Energy - is a form of energy created by
the combined internal motion of atoms in
a substance.
On Earth, there are fundamentally
only three ways in which energy can be transferred
from one place to another: conduction, convection,
and radiation. Conduction involves
the adjacent transfer of heat energy from one atom to
another through the mass of a gas, liquid, or solid.
Condution results in the continuous flow of heat energy
along a temperature gradient from areas of higher to
lower temperature. Convection involves
the transfer of heat energy by way of mass
movements of a substance in gas or liquid form in a
vertical direction (horizontal transfer is called advection).
Convection is
often seen as rising masses of gas or liquid
called convection
currents. It is important to note that energy
transfer by way of conduction and convection depends
on the presence of matter. These forms of energy transfer
do not operate in the vacuum of space. Radiation is
the only means of energy transfer that can occur across
outer space. The transfer of radiation produced at the
Sun's surface through space supplies the Earth with most
of its energy.
Matter: Elements and Compounds
Matter is
the material that makes up things in the Universe. All
matter on the Earth is constructed of elements (see WebElements for
the periodic
table of elements). Chemists have described approximately
115 different elements. Each of these elements have distinct
chemical characteristics. Table 6a-1 lists some
of the chemical characteristics for 48 common elements
found in the Earth's continental
crust.
The smallest particle that exhibits the
unique chemical characteristics of an element is known
as an atom.
Atoms are composed of yet smaller particles known as protons, neutrons,
and electrons. A proton is a subatomic
particle that has significant mass and
contributes a single positive electrical charge to an
atom. Neutrons also have significant mass but no electrical
charge. Electrons are extremely light subatomic particles
having a mass that is 1/1840 of a proton. Each electron
also has a negative electrical charge.
Protons and neutrons make up the nucleus of
an atom. As a result, most of an atom's mass is
concentrated in the nucleus. Because protons are positively
charged the nucleus has a positive charge equal to the
number of these subatomic particles. Electrons are found
orbiting outside the nucleus at various distances based
on their energy level. The area occupied by the electrons
has a negative charge equal to the number of these subatomic
particles. If an atom has an equal number of electrons
and protons its net electrical charge is zero. If there
are more electrons than protons the charge of the atom
is negative. Likewise, if there are less electrons than
protons the charge of the atom is positive. In both cases,
the exact charge is determined by subtracting protons
from electrons. As a result, 4 protons minus 6 electrons
give an atomic charge of -2.
The number of protons found in the nuclei
of the different types of elements is unique and is referred
to as the atomic
number (Table 6a-1). All atoms of a specific
element have the same number of protons in their nuclei. Atomic
mass number is an atom's total number of neutrons
and protons. Many elements have unequal numbers of neutrons
and protons in their nucleus. An element's atomic
weight refers to the total weight of neutrons,
protons, and electrons. For example, the atomic weight
of aluminum is 26.98 (Table 6a-1). Atomic number
describes the number of protons found in an atom. For
example, silver has an atomic number of 47 or 47 protons
in its atom (Table 6a-1). Some elements can have
variants containing different numbers of neutrons but
similar numbers of protons. We call these variants isotopes.
Carbon has two isotopes. Its most common form is carbon-12
which has 6 protons plus 6 neutrons. About 99% of the
carbon on our planet is of this type. The isotope carbon-13
has 6 protons plus 7 neutrons. Carbon-14 is the rarest
isotope of carbon containing 8 neutrons. Some isotopes
are unstable and their nucleus tends to lose subatomic
particles forming an element with a lower atomic mass.
This process is known as radioactive
decay.
| Table
6a-1: Characteristics
of some of the common chemical elements found in
the Earth's continental crust. |
| Element |
Chemical
Symbol
|
Atomic
Number
|
Common
Atomic
Mass
Number
|
Atomic
Weight
|
Percent in
Continental
Crust
|
Required
for all
Life
|
Required
for
Some
Lifeforms
|
Element
Type
|
Moderately
Toxic
|
Extremely
Toxic
|
| Aluminum |
Al
|
13
|
27
|
26.98
|
8.2300
|
-
|
X
|
Metalloid
|
-
|
-
|
| Antimony |
Sb
|
51
|
122
|
121.75
|
0.00002
|
-
|
-
|
Metalloid
|
-
|
-
|
| Arsenic |
As
|
33
|
75
|
74.92
|
0.00018
|
-
|
-
|
Metalloid
|
-
|
X
|
| Barium |
Ba
|
56
|
137
|
137.34
|
0.0425
|
-
|
-
|
Metal
|
-
|
-
|
| Beryllium |
Be
|
4
|
10
|
9.01
|
0.00028
|
-
|
-
|
Metal
|
-
|
X
|
| Bismuth |
Bi
|
83
|
209
|
208.98
|
0.000017
|
-
|
-
|
Metal
|
-
|
-
|
| Boron |
B
|
5
|
11
|
10.81
|
0.0010
|
-
|
-
|
Metalloid
|
-
|
-
|
| Bromine |
Br
|
35
|
80
|
79.91
|
0.00025
|
-
|
-
|
Nonmetal
|
-
|
-
|
| Cadmium |
Cd
|
48
|
112
|
112.40
|
0.00002
|
-
|
-
|
Metal
|
-
|
X
|
| Calcium |
Ca
|
20
|
40
|
40.08
|
4.1000
|
X
|
-
|
Metal
|
-
|
-
|
| Carbon |
C
|
6
|
12
|
12.01
|
0.0200
|
X
|
-
|
Nonmetal
|
-
|
-
|
| Chlorine |
Cl
|
17
|
35.5
|
35.45
|
0.0130
|
-
|
X
|
Nonmetal
|
X
|
-
|
| Chromium |
Cr
|
24
|
52
|
52.00
|
0.0100
|
-
|
-
|
Metal
|
X
|
-
|
| Cobalt |
Co
|
27
|
59
|
58.93
|
0.0025
|
-
|
X
|
Metal
|
-
|
-
|
| Copper |
Cu
|
29
|
63.5
|
63.54
|
0.0055
|
X
|
-
|
Metal
|
X
|
-
|
| Fluorine |
F
|
9
|
19
|
19.00
|
0.0625
|
-
|
X
|
Nonmetal
|
X
|
-
|
| Gallium |
Ga
|
31
|
70
|
69.72
|
0.0015
|
-
|
-
|
Metal
|
-
|
-
|
| Germanium |
Ge
|
32
|
73
|
72.59
|
0.00015
|
-
|
-
|
Metalloid
|
-
|
-
|
| Gold |
Au
|
79
|
197
|
196.97
|
0.0000004
|
-
|
-
|
Metal
|
-
|
-
|
| Hydrogen |
H
|
1
|
1
|
1.008
|
1.4000
|
X
|
-
|
Nonmetal
|
-
|
-
|
| Iodine |
I
|
53
|
127
|
126.90
|
0.00005
|
-
|
X
|
Nonmetal
|
-
|
-
|
| Iron |
Fe
|
26
|
56
|
55.85
|
5.6000
|
X
|
-
|
Metal
|
-
|
-
|
| Lead |
Pb
|
82
|
207
|
207.19
|
0.00125
|
-
|
-
|
Metal
|
-
|
X
|
| Lithium |
Li
|
3
|
6
|
6.94
|
0.0020
|
-
|
-
|
Metal
|
-
|
-
|
| Magnesium |
Mg
|
12
|
24
|
24.31
|
2.3000
|
X
|
-
|
Metal
|
-
|
-
|
| Manganese |
Mn
|
25
|
55
|
54.94
|
0.0950
|
X
|
-
|
Metal
|
-
|
-
|
| Mercury |
Hg
|
80
|
201
|
200.59
|
0.000008
|
-
|
-
|
Metal
|
-
|
X
|
| Molybdenum |
Mo
|
42
|
96
|
95.94
|
0.00015
|
X
|
-
|
Metal
|
-
|
-
|
| Nickel |
Ni
|
28
|
59
|
58.71
|
0.0075
|
-
|
-
|
Metal
|
-
|
X
|
| Nitrogen |
N
|
7
|
14
|
14.01
|
0.0020
|
X
|
-
|
Nonmetal
|
-
|
-
|
| Oxygen |
O
|
8
|
16
|
16.00
|
46.4000
|
X
|
-
|
Nonmetal
|
-
|
-
|
| Palladium |
Pd
|
46
|
106
|
106.40
|
0.000001
|
-
|
-
|
Metal
|
X
|
-
|
| Phosphorus |
P
|
15
|
31
|
30.97
|
0.1050
|
X
|
-
|
Nonmetal
|
-
|
-
|
| Platinum |
Pt
|
78
|
195
|
195.09
|
0.0000005
|
-
|
-
|
Metal
|
-
|
-
|
| Potassium |
K
|
19
|
39
|
39.10
|
2.1000
|
X
|
-
|
Metal
|
-
|
-
|
| Rubidium |
Rb
|
37
|
85.5
|
85.47
|
0.0090
|
-
|
-
|
Metal
|
-
|
-
|
| Selenium |
Se
|
34
|
79
|
78.96
|
0.000005
|
-
|
X
|
Nonmetal
|
X
|
-
|
| Silicon |
Si
|
14
|
28
|
28.09
|
28.2000
|
-
|
-
|
Metalloid
|
-
|
-
|
| Silver |
Ag
|
47
|
108
|
107.87
|
0.000007
|
-
|
-
|
Metal
|
-
|
X
|
| Sodium |
Na
|
11
|
23
|
22.99
|
2.4000
|
-
|
X
|
Metal
|
-
|
-
|
| Sulfur |
S
|
16
|
32
|
32.06
|
0.0260
|
X
|
-
|
Nonmetal
|
-
|
-
|
| Thorium |
Th
|
90
|
232
|
232.04
|
0.00096
|
-
|
-
|
-
|
-
|
-
|
| Tin |
Sn
|
50
|
119
|
118.69
|
0.00020
|
-
|
-
|
Metal
|
X
|
-
|
| Titanium |
Ti
|
22
|
48
|
47.90
|
0.5700
|
-
|
-
|
Metal
|
-
|
-
|
| Tungsten |
W
|
74
|
184
|
183.85
|
0.00015
|
-
|
-
|
Metal
|
-
|
-
|
| Uranium |
U
|
92
|
238
|
238.03
|
0.00027
|
-
|
-
|
-
|
-
|
-
|
| Vanadium |
V
|
23
|
51
|
50.94
|
0.0135
|
-
|
X
|
Metal
|
X
|
-
|
| Zinc |
Zn
|
30
|
65
|
65.37
|
0.0070
|
X
|
-
|
Metal
|
-
|
-
|
Elements can be classified as being either metals, nonmetals,
or metalloids (Table
6a-1). Metals are
elements that usually conduct heat and electricity and
are shiny. Nonmetals do
not conduct electricity that well and are normally not
shiny. Metalloids have
characteristics that are in between metals and nonmetals.
Elements with a net positive or negative
charge are called ions.
Chemists indicate the number of positive or negative
charges on an ion using a superscript after the element's
symbol. For example, calcium has two positive charges
and is written as Ca2+. Some common negatively
charged ions include nitrate (NO3-),
sulfate (SO42-), and phosphate
(PO43-).
Positive and negative ions are electrically
attracted to each other. This mutual attraction allows
for the bonding of atoms to occur forming structures
of matter that are larger than just one atom. When similar
atoms bond together they construct molecules.
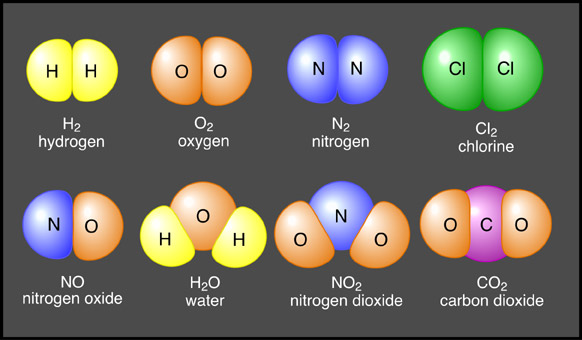
Atoms of different elements joined together form compounds (Figure
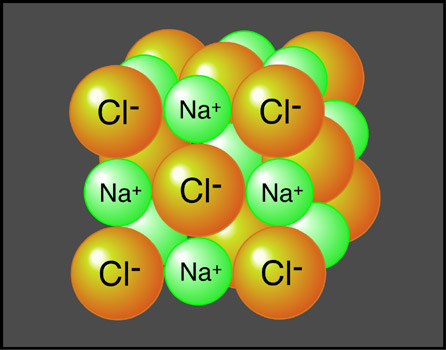
6a-1). Sodium chloride (or table salt), is an ionic
compound consisting of sodium (Na+) and chloride
(Cl-). In nature, it forms as a
three-dimensional array of oppositely charged ions (Figure
6a-2). Many of the Earth's substances have a molecular
structure similar to sodium chloride.
| Figure
6a-1: Some
common molecules and compounds. The molecules in
the top row bond with each other by sharing electrons.
The compounds in the bottom row also share electrons.
However, these joins are called ionic bonds. |
| Figure
6a-2: Atomic
representation of sodium chloride or table salt.
This compound forms in nature as a highly ordered,
three-dimensional network of oppositely charged ions.
The bonds that form between the sodium (Na+)
and (Cl-) chloride ions give
this compound great internal strength allowing it
to form large crystals. |
Inorganic vs. Organic
Compounds and molecules constructed in
living tissues are commonly called organic.
Forms of matter not formed by living things are termed inorganic. Organisms like autotrophs usually create organic
matter by consuming inorganic molecules and compounds
from the lithosphere, hydrosphere, and atmosphere. An
example of an autotroph is any photosynthesizing plant. Heterotrophs consume
and assimilate other
living things to create their organic matter. Herbivores and carnivores are
examples of heterotrophs.
Lipids -
are composed of carbon atoms that have two hydrogen
atoms attached. Lipids are commonly known as fats and
oils, and belong to the family of molecules known as
hydrocarbons.
Carbohydrates -
are composed of carbon, oxygen, and hydrogen atoms.
Some examples are sugars, starch, and cellulose.
Proteins -
are organic compounds that are made primarily of carbon,
hydrogen, nitrogen, and some other minor elements that
are arranged into 20 different compounds known as amino
acids.
Nucleic
Acids - are composed primarily of different
combinations of carbon, hydrogen, nitrogen, oxygen,
and phosphorus. They are very complex compounds
being created by the atomic linking of thousands
of individual atoms. DNA or deoxyribonucleic
acid, the genetic blueprint of life, is
an example of a nucleic acid.
Cells
All organisms are composed of one or more
of cells.
Cells are the smallest self-functioning unit found in
living organisms. Cells are also where the processes
of metabolism and heredity occur
in an organism. Cells arise by the cellular division
of a previously existing cell. Biologists have differentiated
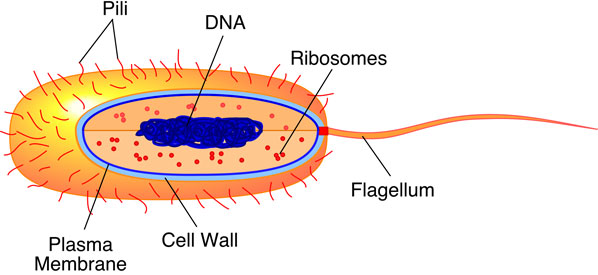
two basic types of cells in organisms. Bacteria, archaea,
and cyanobacteria have
cells that are quite uncomplicated in terms of structure
and function. Quite simply, they lack internal organization.
These cells are commonly known as prokaryotes (Figure
6a-3).
| Figure
6a-3: Typical prokaryote cell.
These cells are about 1 to 10 micrometers in size.
The cell is encased by rigid cell wall and a plasma
membrane. Within the cell, the two most obvious structures
are ribosomes and DNA. The DNA is not bounded by a
membrane. Many prokaryote cells also have a flagellum
which is used for movement. |
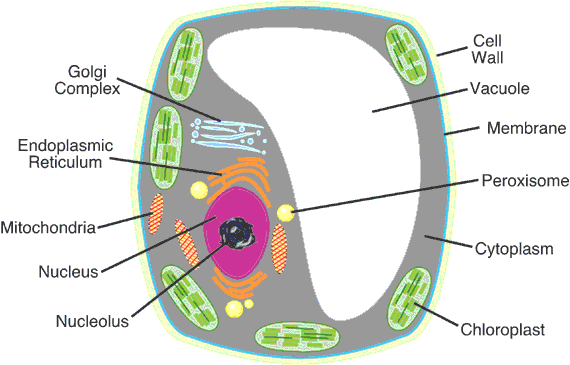
The cells of plants and
animals are more complex than those of bacteria, archaea,
and cyanobacteria. We call these type of cells eukaryotes.
Eukaryotic cells have a membrane enclosed nucleus which
contains the organism's DNA. Plant and animal cells
also contain a variety of membrane-bound structures known
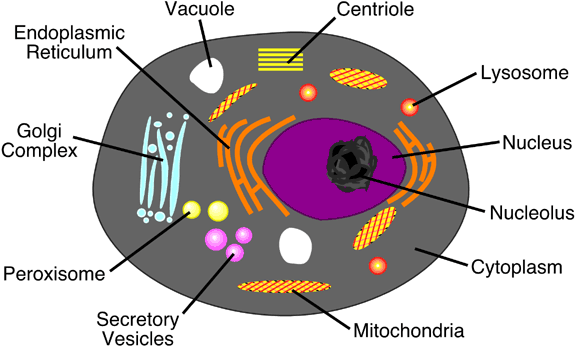
as organelles. Figures 6a-4 and 6a-5 describe
the various structures found in typical plant and animals
cells.
Eukaryotic cells also show great variation
in terms of size. White blood cells of mammals are some
of the smallest eukaryotic cells with a diameter between
3 to 4 micrometers. Ostrich ovum are very large cells
with a diameter of about 100 micrometers.
|
Figure
6a-4: Typical plant cell. Plants
cells differ from animal cells in the following
ways: they have a cell wall, chloroplasts,
and they often contain a large central vacuole.
|
|
Figure
6a-5: Typical animal cell.
|
Internally, cells contain specialized structures
known as organelles that carry out distinct
cellular functions. Within these structures enzymes,
a type of protein, are used to facilitate and regulate
various chemical reactions. Table 6a-2 describes
the function of a variety of cell structures including
many organelles.
|
Table
6a-2: Description and function of
common cell structures.
|
| Structure |
Description |
Function |
| Cell Wall |
Outer layer on a cell composed of cellulose
or other complex carbohydrates. |
Helps to support and protect the cell. |
| Plasma Membrane |
A layer composed of lipids and proteins
that controls the permeability of the cell to water
and dissolved substances. |
Regulates the movement of material
into and out of the cell. |
| Flagellum (Flagella pl.) |
Threadlike organelle that extends from
the surface of the cell. Found in both prokaryotes
and eukaryotes. |
Used for movement of the cell or to
move fluids over the cell's surface for absorption. |
| Pilus (Pili pl.) |
Hollow, hairlike structures made of
protein found on prokaryote cells. |
Allows cell to attach itself to another
cell. |
| Ribosomes |
Tiny, complex structures composed of
protein and RNA. Often attached to endoplasmic reticulum. |
Ribosomes are involved in protein synthesis. |
| Endoplasmic Reticulum |
Extensive system of internal membranes. |
Forms compartments to isolate cell
substances. |
| Nucleus |
Double membrane structure that encases
chromosomes. |
Cell
structure which
directs protein synthesis and cell reproduction. |
| Chromosomes |
Long strands of DNA. |
Store hereditary information. |
| Nucleolus |
Aggregations of rRNA and ribosomal
proteins. |
Area were ribosomes are manufactured. |
| Golgi Complex |
Flattened stacks of membranes. |
Used in the collection, packaging,
and distribution of synthesized molecules. |
| Peroxisomes |
Membrane confined spherical body about
0.2 to 0.5 micrometers in diameter. |
Formed by the endoplasmic reticulum.
Converts fats into carbohydrates. Detoxifies potentially
harmful oxidants. |
| Lysosomes |
Membrane confined spherical body about
0.2 to 0.5 micrometers in diameter. |
Formed by the golgi complex. Contains
digestive enzymes for braking down old cellular components. |
| Centrioles |
Long hollow tubes composed of protein.
Not found in plant cells. |
Influence cell shape, move chromosomes
during reproductive division, and are the internal
structure for flagellum. |
| Secretory Vesicles |
Membrane enclosed sack created at the
golgi complex. |
These structures contain cell secretions,
like hormones and neurotransmitters. The secretory
vesicles are then transported to the cell surface
where they are release to the environment outside
the cell. |
| Vacuole |
Voids within the cytoplasm. Quite large
in plant cells. |
Used to store water and waste products. |
| Cytoplasm |
Semifluid mixture that occupies most
of the cell's interior. Contains sugars, amino acids,
and proteins. Also, contains a protein fiber network. |
Medium in which organelles and other
internal structures exist in. Fiber network support
the shape of the cell and anchor organelles to fixed
positions. |
| Mitochondria |
Elongated structures about 1 to 3 micrometers
long. Resemble aerobic bacteria. |
Structure which converts sugar into
energy through oxidation. |
| Chloroplasts |
Elongated structures with vesicles
containing chlorophyll. |
Site of photosynthesis. |
Cells can also be classified according
to how they obtain their energy. Some cells have the
ability to use light or chemical energy found in the
outside environment to manufacture their own sugars,
fats, and proteins. We call these types of cells autotrophs.
All 400,000 species of plants and a few species of bacteria
use sunlight and the process of photosynthesis to
obtain their energy. Some bacteria breakdown molecules
found in the environment to release chemical energy to
sustain their life. Organisms can also obtain their energy
by consuming other organisms. These organisms are called heterotrophs.
Heterotrophs include most types of bacteria and all of
the animal and fungi species.
Some organisms consist of just one cell
(bacteria, algae,
and protozoa). However, most organisms
are multi-cellular.
Within multi-cellular organisms, groups of cells can
become specialized to carry out a certain function. We
call these functional groups of cells a tissue.
An organ is
a structure composed of several different types of tissues.
Organs also have a specific structure and a particular
function
|
|
No comments:
Post a Comment