Ozone is a gas made up of three oxygen atoms (O3). It occurs naturally in small (trace) amounts in the upper atmosphere (the stratosphere). Ozone protects life on Earth from the Sun’s ultraviolet (UV) radiation. In the lower atmosphere (the troposphere) near the Earth’s surface, ozone is created by chemical reactions between air pollutants from vehicle exhaust, gasoline vapors, and other emissions. At ground level, high concentrations of ozone are toxic to people and plants.
Stratospheric “good” ozone
Ninety percent of the ozone in the atmosphere sits in the stratosphere, the layer of atmosphere between about 10 and 50 kilometers altitude. The natural level of ozone in the stratosphere is a result of a balance between sunlight that creates ozone and chemical reactions that destroy it. Ozone is created when the kind of oxygen we breathe—O2—is split apart by sunlight into single oxygen atoms. Single oxygen atoms can re-join to make O 2, or they can join with O 2 molecules to make ozone (O 3). Ozone is destroyed when it reacts with molecules containing nitrogen, hydrogen, chlorine, or bromine. Some of the molecules that destroy ozone occur naturally, but people have created others.The total mass of ozone in the atmosphere is about 3 billion metric tons. That may seem like a lot, but it is only 0.00006 percent of the atmosphere. The peak concentration of ozone occurs at an altitude of roughly 32 kilometers (20 miles) above the surface of the Earth. At that altitude, ozone concentration can be as high as 15 parts per million (0.0015 percent).
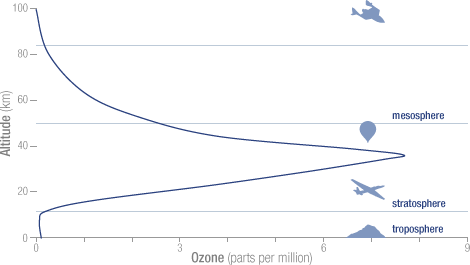 The concentration of ozone varies with altitude. Peak
concentrations, an average of 8 molecules of ozone per
million molecules in the atmosphere, occur between an
altitude of 30 and 35 kilometers.
The concentration of ozone varies with altitude. Peak
concentrations, an average of 8 molecules of ozone per
million molecules in the atmosphere, occur between an
altitude of 30 and 35 kilometers.
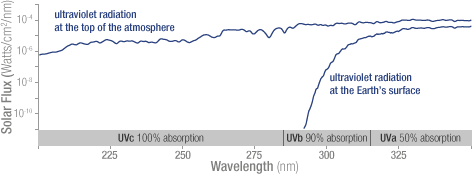 Solar ultraviolet radiation is largely absorbed by the ozone
in the atmosphere—especially the harmful, high-energy
UV-a and UV-b. The graph shows the flux (amount of energy
flowing through an area) of solar ultraviolet radiation at
the top of the atmosphere (top line) and at the
Earth’s surface (lower line). The flux is shown on a
logarithmic scale, so each tick mark on the y-axis indicates
10 times more energy.
Solar ultraviolet radiation is largely absorbed by the ozone
in the atmosphere—especially the harmful, high-energy
UV-a and UV-b. The graph shows the flux (amount of energy
flowing through an area) of solar ultraviolet radiation at
the top of the atmosphere (top line) and at the
Earth’s surface (lower line). The flux is shown on a
logarithmic scale, so each tick mark on the y-axis indicates
10 times more energy.
No comments:
Post a Comment