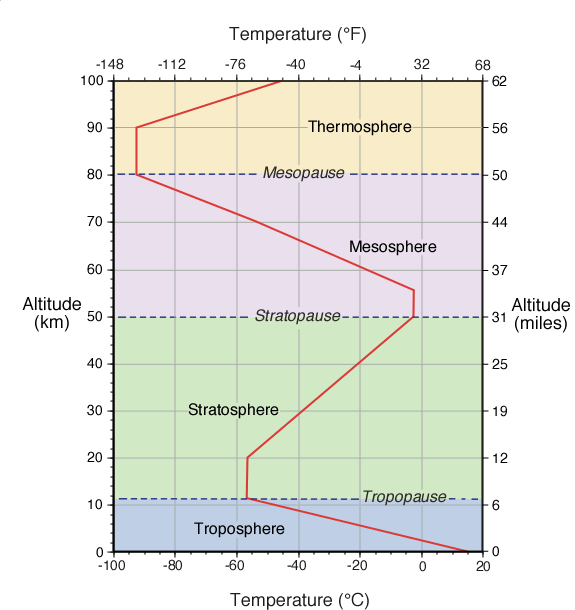
The Earth's atmosphere contains several
different layers that can be defined according to air temperature, Figure
7b-1 displays these layers in an average atmosphere.
| Figure
7b-1: Vertical
change in average global atmospheric temperature.
Variations in the way temperature changes with height
indicates the atmosphere is composed of a number
of different layers (labeled above). These variations
are due to changes in the chemical and physical characteristics
of the atmosphere with altitude. |
According to temperature, the atmosphere
contains four different layers (Figure 7b-1).
The first layer is called the troposphere.
The depth of this layer varies from about 8 to 16 kilometers.
Greatest depths occur at the tropics where warm temperatures
causes vertical expansion of the lower atmosphere. From
the tropics to the Earth's polar regions the troposphere
becomes gradually thinner. The depth of this layer at
the poles is roughly half as thick when compared to the
tropics. Average depth of the troposphere is approximately
11 kilometers as displayed in Figure 7b-1.
About 80% of the total
mass of the atmosphere is contained in troposphere.
It is also the layer where
the majority of our weather occurs (Figure 7b-2).
Maximum air temperature also occurs near the Earth's
surface in this layer. With
increasing height, air temperature drops uniformly with
altitude at a rate of approximately 6.5° Celsius
per 1000 meters. This phenomenon is commonly called the Environmental
Lapse Rate. At an average temperature of -56.5° Celsius,
the top of the troposphere is reached. At the upper edge
of the troposphere is a narrow transition zone known
as the tropopause.
| Figure
7b-2: Most of our planet's weather
occurs in the troposphere. This image shows
a view of this layer from an airplane's window
(Photo © 2004 Edward
Tsang). |
Above the tropopause is the stratosphere.
This layer extends from an average altitude of 11 to
50 kilometers above the Earth's surface. This stratosphere
contains about 19.9% of the total mass found in the
atmosphere. Very little weather occurs in the stratosphere.
Occasionally, the top portions of thunderstorms breach
this layer. The lower portion of the stratosphere is
also influenced by the polar
jet stream and subtropical
jet stream. In the first 9 kilometers of the
stratosphere, temperature remains constant with height.
A zone with constant temperature in the atmosphere is
called an isothermal
layer. From an altitude of 20 to 50 kilometers,
temperature increases with an increase in altitude. The
higher temperatures found in this region of the stratosphere
occurs because of a localized concentration of ozone gas
molecules. These molecules absorb ultraviolet sunlight
creating heat energy that warms the stratosphere. Ozone
is primarily found in the atmosphere at varying concentrations
between the altitudes of 10 to 50 kilometers. This layer
of ozone is also called the ozone
layer . The ozone layer is important to organisms
at the Earth's surface as it protects them from the harmful
effects of the Sun's ultraviolet radiation. Without the
ozone layer life could not exist on the Earth's surface.
Separating the mesosphere from
the stratosphere is transition zone called the stratopause.
In the mesosphere, the atmosphere reaches its coldest
temperatures (about -90° Celsius) at a height of
approximately 80 kilometers. At the top of the mesosphere
is another transition zone known as the mesopause.
The
last atmospheric layer has an altitude greater than
80 kilometers and is called the thermosphere.
Temperatures in this layer can be greater than
1200° C. These high temperatures are generated
from the absorption
of intense solar radiation by oxygen molecules
(O2).
While these temperatures seem extreme, the amount of
heat energy involved is very small. The amount of heat
stored in a substance is controlled in part by its
mass. The air in the thermosphere is extremely thin
with individual gas molecules being separated from
each other by large distances. Consequently, measuring
the temperature of thermosphere with a thermometer
is a very difficult process. Thermometers measure
the temperature of bodies via the movement of heat
energy. Normally, this process takes a few minutes
for the conductive transfer of kinetic energy from
countless molecules in the body of a substance to the
expanding liquid inside the thermometer. In the
thermosphere, our thermometer would lose more heat
energy from radiative emission then what it would gain
from making occasional contact with extremely hot gas
molecules.

No comments:
Post a Comment