In the stratosphere, the region of the atmosphere
between about 6 and 30 miles (10 and 50 kilometers) above the
Earth's surface, ozone (O3) plays a vital
role by absorbing harmful ultraviolet radiation from the sun.
Stratospheric ozone is threatened by some of the human-made
gases that have been released into the atmosphere, including
those known as chlorofluorocarbons (CFCs). Once widely used
as propellants in spray cans, refrigerants, electronics cleaning
agents, and in foam and
insulating products, the CFCs had been hailed
as the "wonder chemicals." But the very properties
that make them useful - chemical inertness, non-toxicity, insolubility
in water - also make them resistant to removal in the lower
atmosphere.
CFCs are mixed worldwide by the large-scale motions
of the atmosphere and survive until, after 1-2 years, they reach
the stratosphere and are broken down by ultraviolet radiation.
The chlorine atoms within them are released and directly attack
ozone. In the process of destroying ozone, the chlorine atoms
are regenerated and begin to attack other ozone molecules...
and so on, for thousands of cycles before the chlorine atoms
are removed from the stratosphere by other processes.
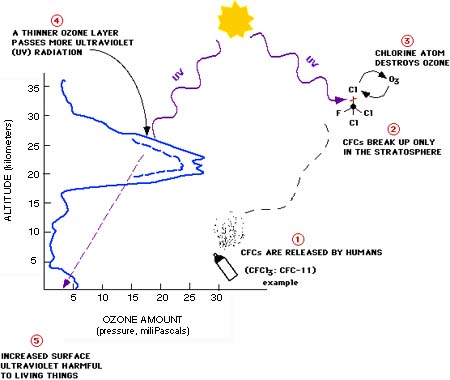
The profile above shows how the amount of
ozone (O3) varies with height in the
atmosphere. Note that most of the ozone is in the lower stratosphere.
The "ozone layer" resides at an altitude of about
12 to 15 miles (20 to 25 kilometers) above sea level. It acts
as a shield by absorbing biologically active ultraviolet light
(called UV-B) from the sun. If the ozone layer is depleted,
more of this UV-B radiation reaches the surface of the earth.
Increased exposure to UV-B has harmful effects on plants and
animals, including humans. The chlorine and bromine in human-produced
chemicals such as the ones known as chlorofluorocarbons (CFCs)
and halons are depleting ozone in the stratosphere. The figure
shows a simplified cycle of reactions in which chlorine (Cl)
destroys ozone (O3).
No comments:
Post a Comment