Converting air pollutant concentrations
The conversion equations depend on the temperature at which the conversion is wanted (usually about 20 to 25 degrees Celsius). At an ambient air pressure of 1 atmosphere (101.325
kPa), the general equation is:
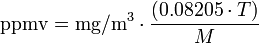
and for the reverse conversion:
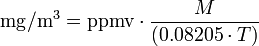
| where: |
|
| ppmv |
= air pollutant concentration, in parts per million by volume |
| mg/m³ |
= milligrams of pollutant per cubic meter of air |
 |
= atmospheric temperature in kelvins = 273.15 + °C |
| 0.08205 |
= Universal Gas Law constant in atm·l/(mol·K) |
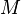 |
= molecular weight of the air pollutant (dimensionless) |
Notes:
- Pollution regulations in the United States typically reference
their pollutant limits to an ambient temperature of 20 to 25 °C as
noted above. In most other nations, the reference ambient temperature
for pollutant limits may be 0 °C or other values.
- 1 percent by volume = 10,000 ppmv (i.e., parts per million by volume).
- atm = absolute atmospheric pressure in atmospheres
- mol = gram mole

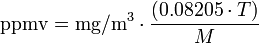
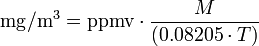
No comments:
Post a Comment