Definition of pH
This
discovery definitely marked a great step forward. However, since
activity cannot be determined by actual direct measurement, it cannot be
used as the basis for theoretical calculation of pH value. Accordingly,
actual pH is determined by comparison with the pH of a specific
solution, a standard solution that tends to maintain its pH value.
Methods for measuring pH are defined by ISO and JIS.
JIS (Japanese Industrial Standards) Regulations
When
pH measurement became commonplace, differences between values measured
for the same sample emerged as a problem. As a result, it became
necessary to give a clear definition of pH and establish a method for
selecting standard solutions. Hence, it became a pressing requirement
that an effort be made to establish JIS regulations for pH measurement
methods as soon as possible. Through various types of research and
study, and with the cooperation of various types of organizations, JIS
for pH measurement was established in March, 1957.
When drafting the JIS, the authors also referred to the standards for pH measurement in the United States, United Kingdom, and France. This is because the unit of pH is used internationally as well as in Japan. If individual countries created independent definitions, these would be unacceptable as international standards and would cause problems for academic and commercial activity.
When drafting the JIS, the authors also referred to the standards for pH measurement in the United States, United Kingdom, and France. This is because the unit of pH is used internationally as well as in Japan. If individual countries created independent definitions, these would be unacceptable as international standards and would cause problems for academic and commercial activity.
For more detailed information on pH, please see the references below.
The following page gives an unmodified excerpt of part of “JIS Z 8802: Methods for determination of pH of aqueous solutions”. The JIS were established for industrial use. In order to gain a further, more precise understanding of pH, we recommend you to read - or reread - them through.
Kameyama: “Denki-kagaku no Riron oyobi Ouyou—Joukan I (Theory and Applications of Electrochemistry, First Volume I),” Maruzen Co., Ltd., 1963
Yoshimura, Matsushita, Morimoto: “pH no Riron to Sokutei-hou (Theory of pH and its Methods of Measurement),” Maruzen Co., Ltd., 1968
Kishimoto, Matsushita: “pH Sokutei to Jidouseigyo (pH Measurement and Automatic Control),” Nikkan Kogyo Shimbun, Ltd., 1968
Bates: “Determination of pH,” John Wiley & Sons, 1964
Ives, Janz: “Reference Electrodes,” Academic Press, 1961
“JIS Z 8802: Methods for determination of pH of aqueous solutions,” Japanese Standards Association
“JIS Z 8805: Glass electrodes for measurement of pH,” Japanese Standards Associatio
The following page gives an unmodified excerpt of part of “JIS Z 8802: Methods for determination of pH of aqueous solutions”. The JIS were established for industrial use. In order to gain a further, more precise understanding of pH, we recommend you to read - or reread - them through.
Kameyama: “Denki-kagaku no Riron oyobi Ouyou—Joukan I (Theory and Applications of Electrochemistry, First Volume I),” Maruzen Co., Ltd., 1963
Yoshimura, Matsushita, Morimoto: “pH no Riron to Sokutei-hou (Theory of pH and its Methods of Measurement),” Maruzen Co., Ltd., 1968
Kishimoto, Matsushita: “pH Sokutei to Jidouseigyo (pH Measurement and Automatic Control),” Nikkan Kogyo Shimbun, Ltd., 1968
Bates: “Determination of pH,” John Wiley & Sons, 1964
Ives, Janz: “Reference Electrodes,” Academic Press, 1961
“JIS Z 8802: Methods for determination of pH of aqueous solutions,” Japanese Standards Association
“JIS Z 8805: Glass electrodes for measurement of pH,” Japanese Standards Associatio
Meaning of pH
In
this standard, pH means a value determined based on the definition of
the pH scale. It does not have any strict physical and chemical meaning.
Notes: A particular solution, such as a buffer solution
with less than 0.1 mol/l with pH ranging from 3 to 10 is assumed to
be as shown in formula(1)
Definition of the pH Scale
When
representing the pH values of two solutions, with solution X and
solution S at the same temperature, for pH (X) and pH (S), the
difference between those pH values is defined by formula (2)
where
Ex is the electromotive force of a battery with a glass electrode and
reference electrode placed in solution X, and Es is the electromotive
force of a battery with a glass electrode and reference electrode placed
in solution S.
R: gas constant of 8.3144J/.C¥ mol
T: absolute temperature of t.C+ 273.15
F: Faraday constant of 96495 C/g-equiv.
In formula (2), the same units of measure must be used in the denominator and numerator.Table 1 shows values for 2.3026 RT/F in mV at each temperature.
R: gas constant of 8.3144J/.C¥ mol
T: absolute temperature of t.C+ 273.15
F: Faraday constant of 96495 C/g-equiv.
In formula (2), the same units of measure must be used in the denominator and numerator.Table 1 shows values for 2.3026 RT/F in mV at each temperature.
Table 1--Values of 2.3026RT/F
Temp(.C)
|
2.3026RT/F mV
|
Temp(.C)
|
2.3026RT/F mV
|
|---|---|---|---|
0
|
54.19
|
50
|
64.11
|
5
|
55.19
|
55
|
65.11
|
10
|
56.18
|
60
|
66.10
|
15
|
57.17
|
65
|
67.09
|
20
|
58.16
|
70
|
68.08
|
25
|
59.15
|
75
|
69.07
|
30
|
60.15
|
80
|
70.07
|
35
|
61.14
|
85
|
71.06
|
40
|
62.13
|
90
|
72.05
|
45
|
63.12
|
95
|
73.04
|
The
definition represented by formula (2) means that the pH of all
solutions are measured in reference to the known pH of a reference
solution. That solution is 0.05 mol/l phthalate solution. the pH of
which is 4.000 at 15℃
In
other words, a glass electrode is devised to generate accurate
electromotive force due to the difference in pH. And a reference
electrode is devised not to cause electromotive force due to a
difference in pH
Principles of the Glass-Electrode Method
In
the glass-electrode method, the known pH of a reference solution is
determined by using two electrodes, a glass electrode and a reference
electrode, and measuring the voltage (difference in potential) generated
between the two electrodes. The difference in pH between solutions
inside and outside the thin glass membrane creates electromotive force
in proportion to this difference in pH. This thin membrane is called the
electrode membrane. Normally, when the temperature of the solution is
30 ℃, if the pH inside is different from that of outside by 1, it will
create approximately 60 mV of electromotive force.
The liquid inside the glass electrode usually has a
pH of 7. Thus, if one measures the electromotive force generated at the
electrode membrane, the pH of the sample can be found by calculation.
A second electrode is necessary when measuring the electromotive force generated at the electrode membrane of a glass electrode. This other electrode, paired with the glass electrode, is called the reference electrode. The reference electrode must have extremely stable potential. Therefore, it is provided with a pinhole or a ceramic material at the liquid junction.
A second electrode is necessary when measuring the electromotive force generated at the electrode membrane of a glass electrode. This other electrode, paired with the glass electrode, is called the reference electrode. The reference electrode must have extremely stable potential. Therefore, it is provided with a pinhole or a ceramic material at the liquid junction.
Detector (Glass Electrode)
Glass Electrode
A
glass electrode consists of an electrode membrane that responds to pH, a
highly isolating base material to support the unit, solution inside the
glass electrode, an internal electrode, a lead wire, and a glass
electrode terminal.
The most critical item in this system is the electrode membrane. First, the membrane glass must generate a potential that accurately corresponds to the pH of the solution. Second, even though it must be accurately sensitive to acidity and alkalinity, it must not be damaged by them. Third, the electric resistance of the membrane itself must not be too large. Fourth, too large a difference in potential (asymmetric difference in potential) must not be generated between the solutions inside and outside the electrode when the electrode is immersed in a solution of identical pH to that of the solution inside of the electrode. Another requirement is that the glass membrane be resistant to shock and chemical reactions.
The most critical item in this system is the electrode membrane. First, the membrane glass must generate a potential that accurately corresponds to the pH of the solution. Second, even though it must be accurately sensitive to acidity and alkalinity, it must not be damaged by them. Third, the electric resistance of the membrane itself must not be too large. Fourth, too large a difference in potential (asymmetric difference in potential) must not be generated between the solutions inside and outside the electrode when the electrode is immersed in a solution of identical pH to that of the solution inside of the electrode. Another requirement is that the glass membrane be resistant to shock and chemical reactions.
Generally, silver chloride is used as the material
for the internal electrode. Potassium chloride solution maintained at pH
7 is usually used as the internal solution.

- Principle figure of glass electrode method
Birth and History of the Glass Electrode
In
1906, Cremer blew the tip of a glass tube into a bubble and measured
the difference in potential between two kinds of solutions (0.6% NaCl +
diluted H2SO4 and O.6% NaCl + diluted NaOH). This
is considered the birth of the glass electrode. In 1909, Habert and
Klemensiewicz measured the difference in potential between a silver
chloride electrode and a mercurous chloride electrode, and found that
they could obtain a titration curve similar to that of a hydrogen
electrode. They called this a glass electrode. So, the glass electrode
took its first step toward becoming a practical pH electrode. However,
early glass electrodes had large electrical resistance and very thin
glass membranes. Therefore, they were very fragile and difficult to
handle.
Later, with the introduction of glass containing lithium, which is chemically strong and has low electric resistance and with development of technology for fabricating electronic parts and insulation materials, the glass electrode made rapid progress after the Second World War. Now it is widely used as the standard for measuring pH.
Later, with the introduction of glass containing lithium, which is chemically strong and has low electric resistance and with development of technology for fabricating electronic parts and insulation materials, the glass electrode made rapid progress after the Second World War. Now it is widely used as the standard for measuring pH.

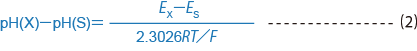


No comments:
Post a Comment