Sample Measurement for low
Conductivity Liquid/Low Buffer Capacity
The
sample measurement for the low buffer capacity/low conductivity liquid
such as pure water, cleaning solution for semiconductors, boiler water,
tap water, groundwater, river water and precipitation is extremely
difficult to perform. The pH indication because unstable or there is no
reproducibility in the measurement..
- Because the pH buffer capacity of the sample is extremely low, the response to the pH of pH–response glass membrane many become slower.
- For the same reason, the contamination of minor constituents (such as measuring containers, constituents in the air ) may affect the pH of the sample.
- At the liquid junction of the reference electrode, the liquid junction potential or the streaming potential may tend to occur.
- The internal solution to be lost from the reference electrode may affect the pH. To solve these problems as much as possible and make more accurate measurement, the following methods are effective.
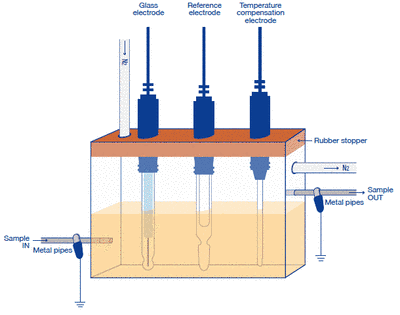
1. Sample measurement in a flow status (flow-through system)
- Prepare a measuring container which has the flow system as shown in the figure.
- Use the glass electrode, the reference electrode (sleeve type or double junction type), the temperature compensation electrode.
- Perform the measurement by setting the whole cell in the shield box.
- Place the reference electrode so as to locate in the downstream in the sample flow.
- Stopper the sample bath securely to perform the nitrogen bubbling.
Because it tales a long time to respond, wait until the indication value becomes stable.
2. Purging by nitrogen or argon gas with the clean airtight container
- Use the glass electrode, the reference electrode (double junction type), the temperature compensation electrode.
- Because electrode noise from outside tend to affect the measurement, perform it in the shield box
- For reducing the influence of the internal solution of the reference electrode, place the sleeve of the reference electrode lower than sensing part of the glass electrode, then stir the sample slowly.
- Stopper the sample container tightly with the lid to avoid exposing the sample to the air, and spray nitrogen to the sample solution level. Then, the influence of carbon dioxide in the air may be reduced.
<Measurement procedure>
(1) Calibrate the glass electrode in the standard solution, and rinse it with pure water.
(2) Fill the measuring container with the sample, and immerse the electrode for more than 10 minutes.(3) Rinse the measuring container and the electrode with new sample (different solution mentioned in (2) above).
(4) Do not wipe the container and electrode of the cleaning liquid out with filter papers or tissue papers. Keep the container and electrode getting wet, fill the sample slowly into the container without bubbling, and measure the sample pH value.
(1) Calibrate the glass electrode in the standard solution, and rinse it with pure water.
(2) Fill the measuring container with the sample, and immerse the electrode for more than 10 minutes.(3) Rinse the measuring container and the electrode with new sample (different solution mentioned in (2) above).
(4) Do not wipe the container and electrode of the cleaning liquid out with filter papers or tissue papers. Keep the container and electrode getting wet, fill the sample slowly into the container without bubbling, and measure the sample pH value.

3. Using proper electrode for the measurement
There are following electrodes for low conductivity water/non-aqueous solvents.
#9630-10D: Quick and stable measurement is possible for low conductivity samples or those with low buffering ability, such as tap water. Suitable for water quality testing at water treatment plants.
#6377-10D: This integrated electrode has good response to aqueous solutions in the low conductivity/low buffer capacity, and to non-aqueous solvents such as alcohol.
#9630-10D: Quick and stable measurement is possible for low conductivity samples or those with low buffering ability, such as tap water. Suitable for water quality testing at water treatment plants.
#6377-10D: This integrated electrode has good response to aqueous solutions in the low conductivity/low buffer capacity, and to non-aqueous solvents such as alcohol.
4. Immerse pH electrode in the sample solution, and rinse with the sample solution

Measurement for strongly
acidic/basic aqueous solution
Current
glass electrodes are resistant to the strongly acidic/basic aqueous
condition. However, if the pH exceeds 12, some error may occur and the
electrode response speed may become slower. Exercise the caution
regarding the following item while performing the measurement.
- Use glass electrode, the reference electrode (sleeve type or double-junction type) and the temperature compensation electrode.
- After the measurement, rinse the sensing part sufficiently with pure water.
- When
the pH is higher than 12, some error (alkaline error) may occur. To
measure the pH value at this range, perform the two-point calibration
with the standard solution of as high the pH as possible (saturated
Ca(OH)2 solution (pH12.45 at 25℃)).
*In this case of measuring a basic sample for the long term, the response speed of the glass electrode may become slower. In this case, immerse the sensing part in 1 mol/L HCl from 10 to 30 minutes, and then rinse it sufficiently with pure water.Measurement of strongly-acidic solutions up to 0.1 N HCl presents no particular problems.
No comments:
Post a Comment